Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Until recently, best practice guidelines recommended withholding rotavirus vaccine in offspring exposed in utero to any TNFi until 6 months of age due to fears of causing infection in immunosuppressed infants. As rotavirus is a common and severe form of gastroenteritis, particularly in unvaccinated infants, delaying vaccine administration until 6 months of age may be associated with more diarrhea-associated morbidity, instead of routine immunization from 2 to 6 months. We compared the risk of diarrhea-associated healthcare events in infants exposed in utero to TNFi according to rotavirus vaccine exposure in the first 6 months of life, examining two specific periods: 2-6 months and 6-24 months.
Methods: Using MarketScan, we created a cohort of offspring born to mothers with chronic inflammatory diseases who received at least one TNFi prescription during the third trimester between 2011 and 2021. We excluded infants born in the 13 US states with a state-funded universal rotavirus vaccine program, as these infants have no private insurer claims for the vaccine. Rotavirus vaccine exposure was identified by ≥1 procedure billing code for RV1 and/or RV5 rotavirus vaccines administered between 2 and 6 months of age; infants vaccinated after 6 months were censored at the vaccine time. For the first analysis, follow-up began at 2 months, when a child could receive their first vaccine, and stopped at 6 months. For the second, follow-up began at 6 months, when a child was theoretically fully vaccinated, until 24 months. The first instance of diarrhea-associated healthcare use was defined by relevant ICD-9/10 diagnostic billing codes for hospitalizations and/or outpatient visits. Cox proportional hazards models estimated hazard ratios (HR) and 95% confidence intervals (CI) for diarrhea-associated healthcare use in infants exposed in utero to TNFi who received the rotavirus vaccine between 2-6 months, compared with those unvaccinated. Models were adjusted for in utero drug exposure, preterm birth, sex, geographic region, year and season of birth, and TNFi placental transfer (high vs low).
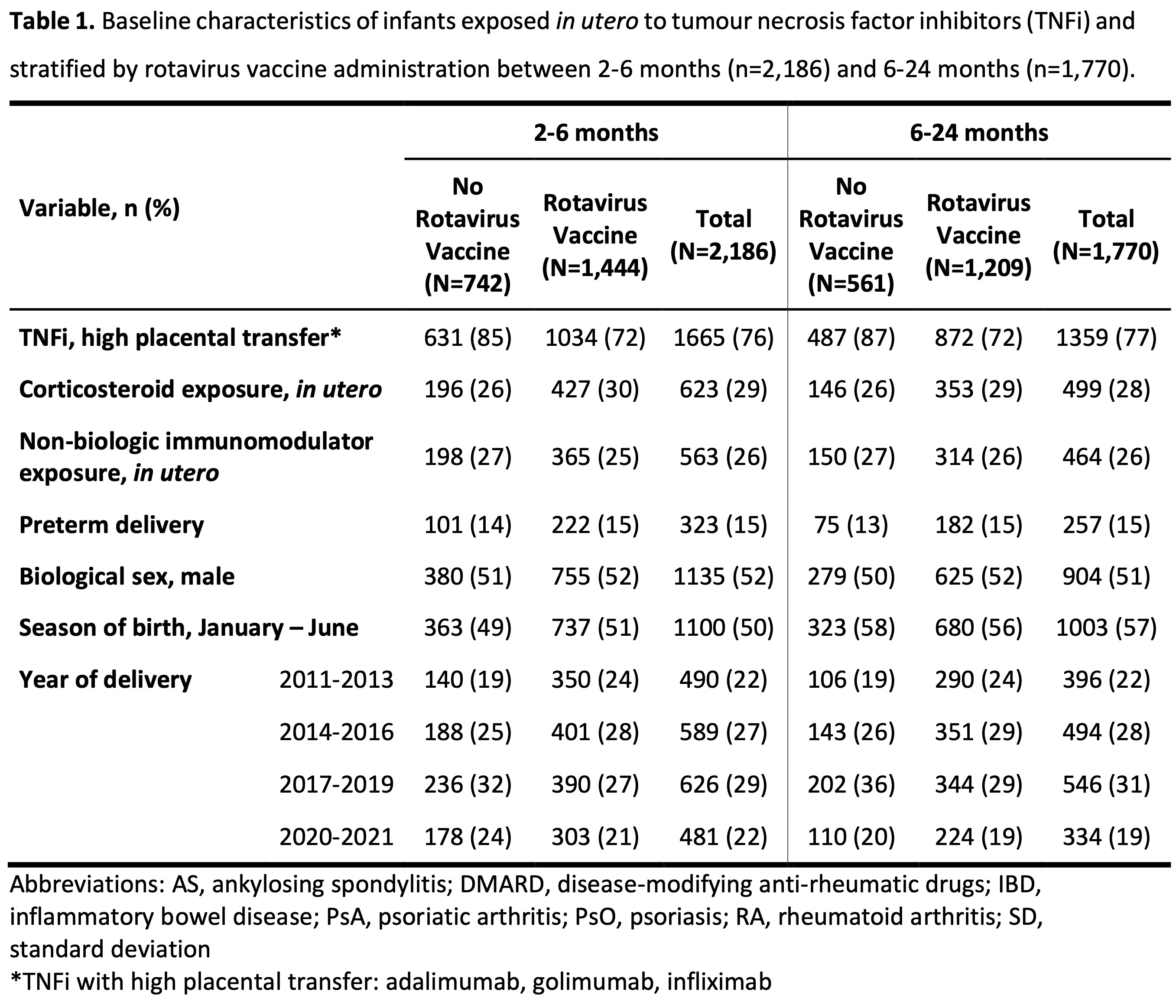
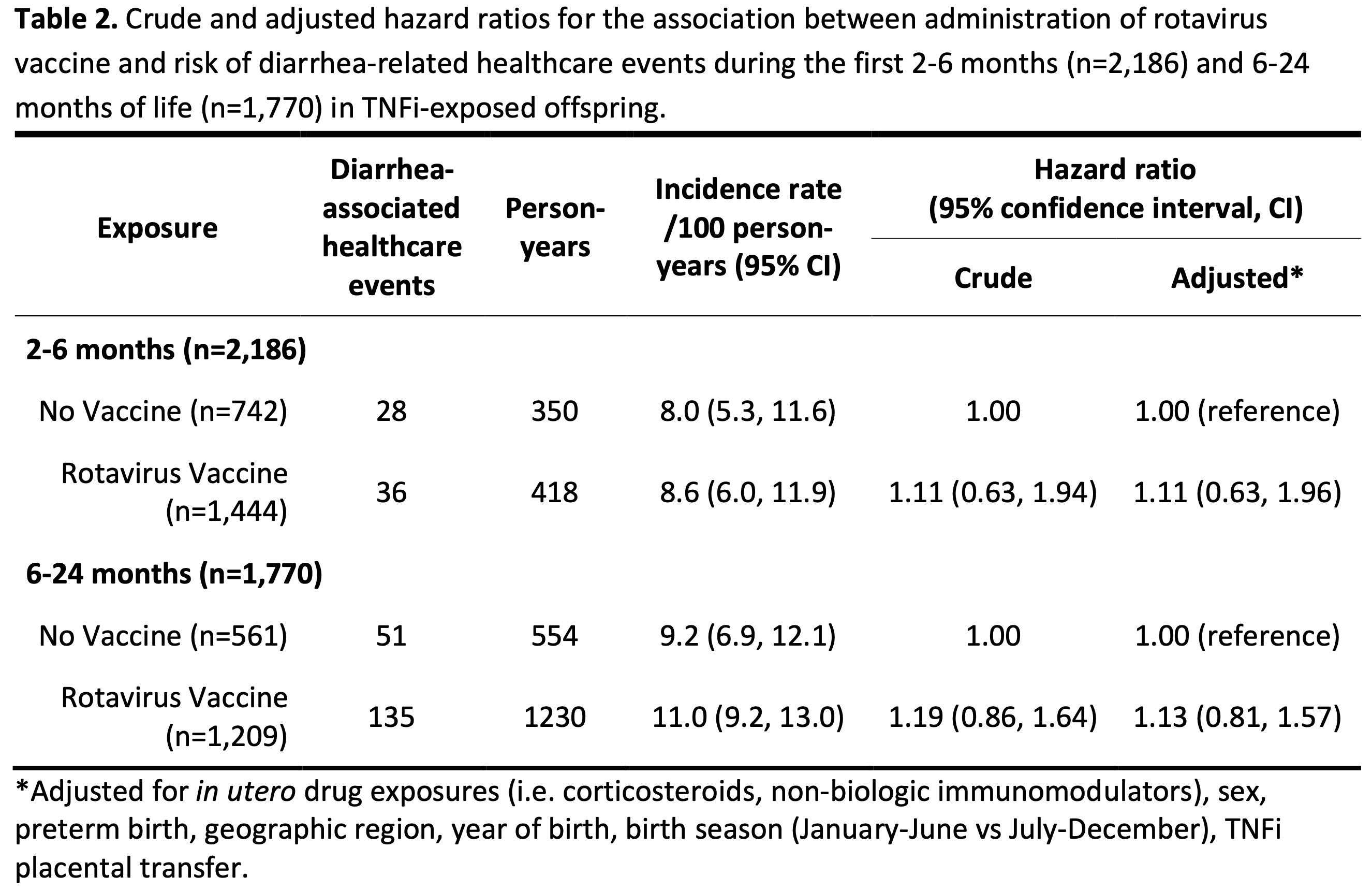
Results: We included 2,186 offspring born to mothers with chronic inflammatory diseases who were exposed in utero to TNFi during the third trimester (Table 1). Of these, 1,444 (66%) received at least one dose of the rotavirus vaccine between 2-6 months of age. Receiving the rotavirus vaccine was not clearly associated with an overall increased risk of diarrhea-associated healthcare events (adjusted HR 1.11; 95% CI 0.63, 1.96) during the first 2-6 months of life compared to no vaccine administration (Table 2). Similarly, receiving the rotavirus vaccine was not associated with a clearly increased risk of diarrhea-associated healthcare events between 6-24 months of life (adjusted HR 1.13; 95% CI 0.81, 1.57) compared to no rotavirus vaccine administration within the first 2-6 months of life.
Conclusion: Our findings indicate no clear increase in diarrhea-associated healthcare events in the months immediately following vaccination and during their first 2 years of life in offspring exposed to TNFi during the third trimester. These results suggest not delaying rotavirus vaccination in TNFi-exposed infants.
To cite this abstract in AMA style:
Flatman L, Bernatsky S, Malhamé I, St-Pierre Y, Basso O, Bérard A, Vinet E. Rotavirus Vaccine in Offspring Exposed to Tumour Necrosis Factor Inhibitors During the Third Trimester Does Not Increase Diarrhea-Associated Healthcare Events [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/rotavirus-vaccine-in-offspring-exposed-to-tumour-necrosis-factor-inhibitors-during-the-third-trimester-does-not-increase-diarrhea-associated-healthcare-events/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rotavirus-vaccine-in-offspring-exposed-to-tumour-necrosis-factor-inhibitors-during-the-third-trimester-does-not-increase-diarrhea-associated-healthcare-events/