Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Romosozumab, an anti-sclerostin antibody that increases bone formation while decreasing bone resorption, reduces fracture risk within 12 months. Here we evaluate the effects of transitioning from denosumab to romosozumab in treatment-naïve patients.
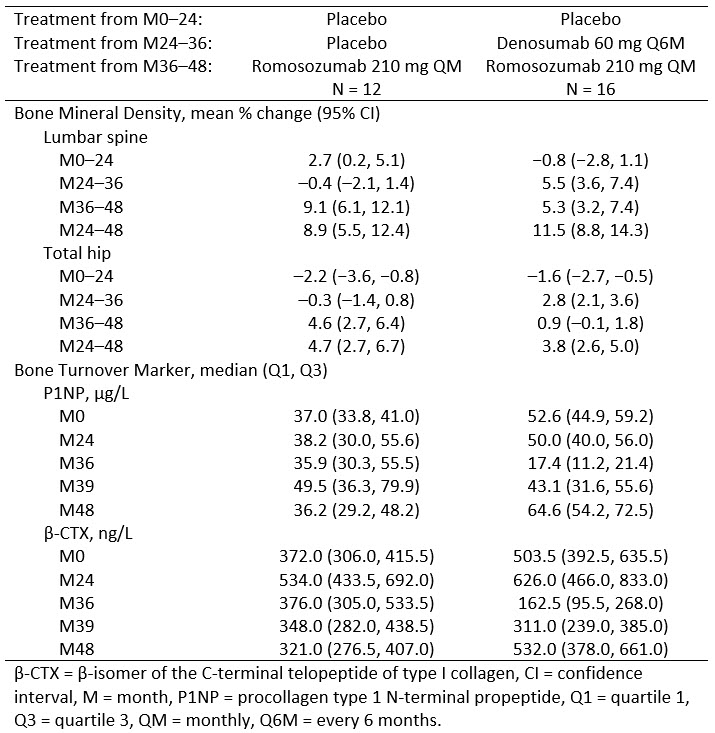
Methods: This phase 2 trial (NCT00896532) enrolled postmenopausal women with a lumbar spine, total hip, or femoral neck T-score ≤ –2.0 and ≥ –3.5. Patients were randomized to placebo or various doses of romosozumab monthly or every 3 months from baseline to month (M) 24, were rerandomized to 12 months of denosumab 60 mg every 6 months or placebo (M24–36), and then all were to receive romosozumab 210 mg monthly for 12 months (M36–48). Results for the overall population have been previously published (McClung et al, J Bone Miner Res 2018; Kendler et al, Osteoporos Int 2019). Here we present data from a subset of patients who were randomized to placebo for 24 months, denosumab (n = 16) or placebo (n = 12) for 12 months, and then romosozumab for 12 months.
Results: In patients who were randomized to placebo followed by denosumab, romosozumab treatment for 12 months maintained BMD gained during denosumab treatment at the total hip (mean change from end of denosumab treatment, 0.9%) and further increased BMD gains at the lumbar spine (mean change from end of denosumab treatment, 5.3%) (Table). As expected, P1NP and CTX levels decreased with denosumab. Upon transition to romosozumab (M36–48), P1NP levels initially increased and gradually returned to baseline by M48 while CTX gradually increased to baseline levels.
In patients who transitioned to romosozumab after 36 months of placebo, BMD increased at the lumbar spine and total hip (Table). P1NP levels initially increased with romosozumab and gradually returned to baseline by M48 while median CTX level remained below baseline with romosozumab treatment.
Conclusion: BMD response in the placebo to romosozumab group was similar to that observed in other studies. Transitioning to romosozumab after 12 months of denosumab further improves lumbar spine BMD and maintains total hip BMD.
To cite this abstract in AMA style:
McClung M, Bolognese M, Brown J, Reginster J, Langdahl B, Ruiz-Santiago N, Shi Y, Rojeski M, Timoshanko J, Libanati C, Kassahun H, Oates M. Romosozumab After Denosumab Improves Lumbar Spine and Maintains Total Hip Bone Mineral Density in Postmenopausal Women with Low Bone Mass [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/romosozumab-after-denosumab-improves-lumbar-spine-and-maintains-total-hip-bone-mineral-density-in-postmenopausal-women-with-low-bone-mass/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/romosozumab-after-denosumab-improves-lumbar-spine-and-maintains-total-hip-bone-mineral-density-in-postmenopausal-women-with-low-bone-mass/