Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
To conduct a cost-effectiveness analysis from a health system perspective of various strategies in managing chronic gout to mitigate risk of allopurinol-induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis, including three that utilize results of HLA-B*5801 genetic testing.
Methods
A decision tree model was developed to estimate costs and quality-adjusted life years (QALYs) over 20-year horizon for patients with chronic gout who fulfil the criteria for initiating ULT with either allopurinol or probenecid. Strategies modelled were: (a) Standard ULT with allopurinol as first-line drug (ULT); (b) Standard ULT with allopurinol as 1st line drug coupled to a safety programme (ULT+SP) that monitors for signs of SJS/TEN; (c) HLA-B*5801 genetic testing-guided ULT treatment (G->ULT) in which choice of 1st line ULT is based on test results (probenecid for test positive, allopurinol for test negative) and avoidance of allopurinol in HLA-B*5801 positive patients; (d) genetic testing to enrol test positive patients in a safety programme when initiating allopurinol (G->SP); test negative patients would receive allopurinol without SP; (e) HLA-B*5801 genetic guided ULT with the safety programme (G->ULT->SP) in which test positive patients are initially given probenecid, but non-responders are subsequently switched to allopurinol in the presence of the safety programme; (f) No ULT and treatment of acute flares only (no ULT). Although inputs are based on the Singapore context, the model is a general template that can be readily adapted to other populations and countries.
Results
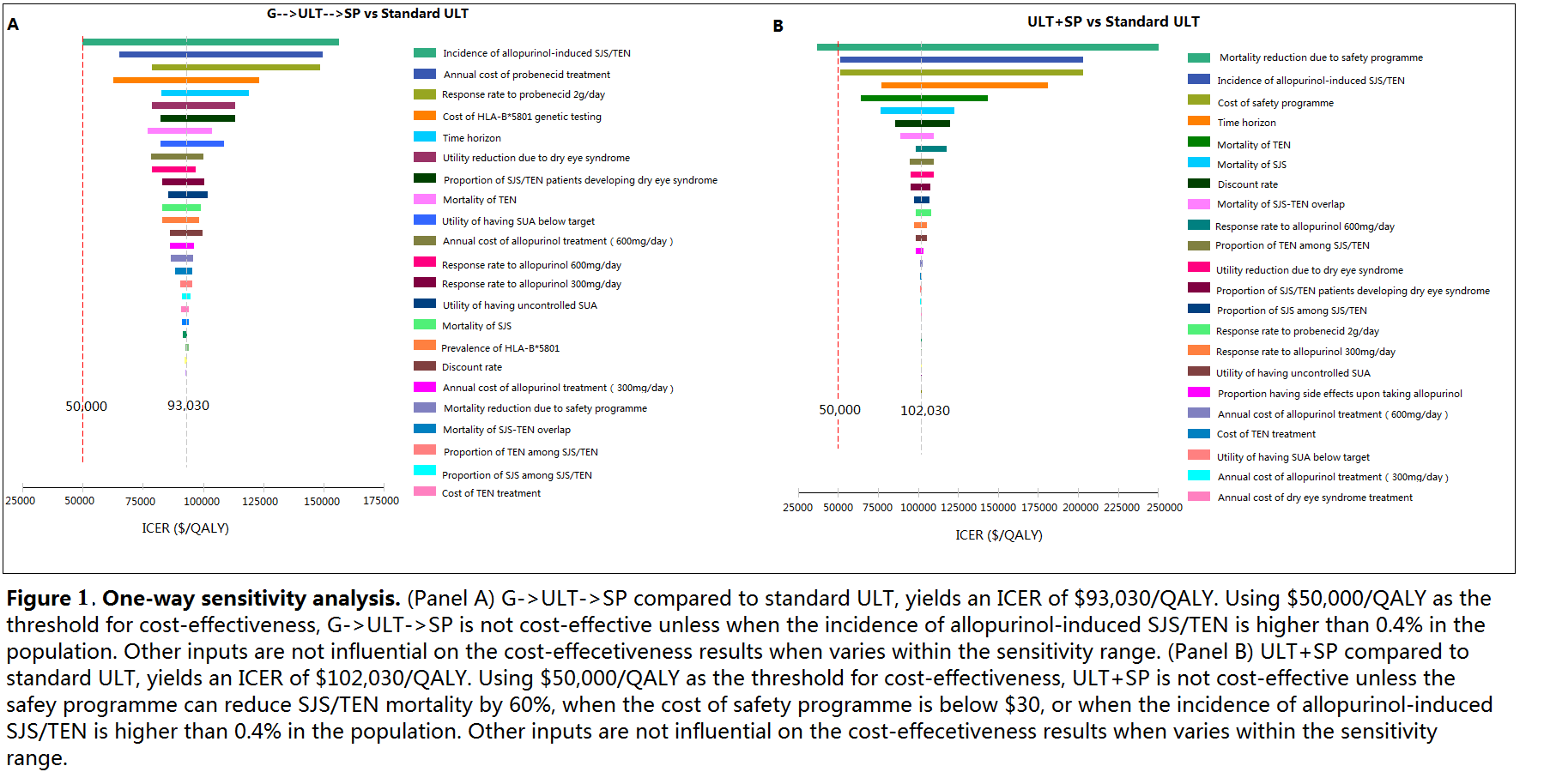
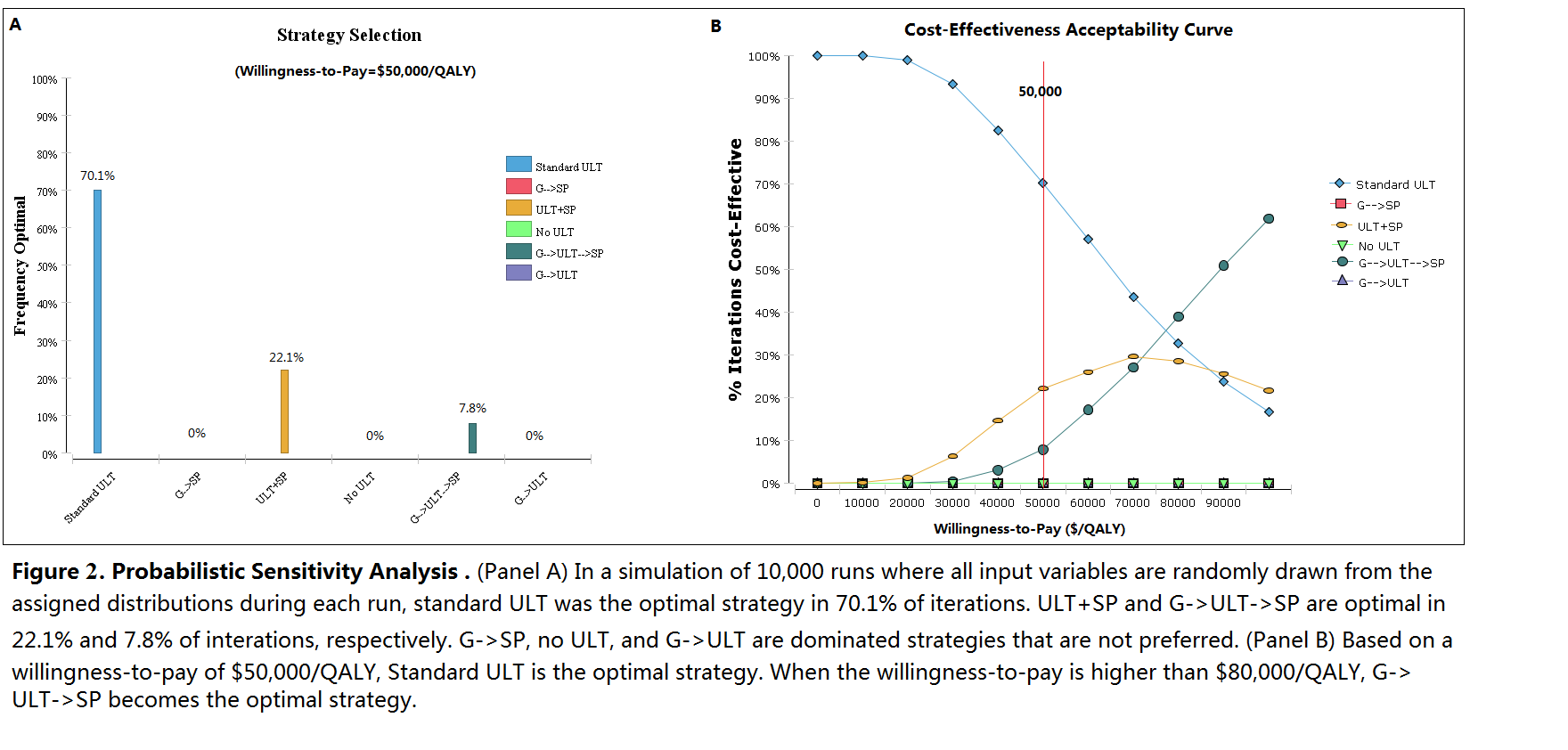
No ULT and treating acute flares only has the lowest QALYs and highest costs. Compared with standard ULT, G->ULT increases cost by US$910 but reduces QALYs, despite the reduction in SJS/TEN risk. ULT+SP has an incremental cost-effectiveness ratio (ICER) of US$102,030/QALY compared to ULT alone. G->SP achieves the same QALYs as ULT+SP but at higher cost. G->ULT->SP results in an ICER of $93,030/QALY compared to standard ULT. (Figure 1 and 2)
Conclusion
Standard care with allopurinol as first line treatment for chronic gout without genetic testing remains the optimal strategy from a cost-effectiveness perspective based on a threshold of US$50,000/QALY. A safety programme for all patients is not cost-effective, but may become so if implementation costs decrease. If genetic testing costs decrease, testing may become cost-effective if results are used to guide the selection of 1st line ULT, with allopurinol as 2nd line ULT for HLA-B*5801 positive patients in the presence of a safety programme.
Disclosure:
D. Dong,
None;
W. C. Tan-Koi,
None;
G. G. Teng,
None;
E. Finkelstein,
None;
C. Sung,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/role-of-hla-b5801-genetic-testing-and-a-safety-programme-when-initiating-allopurinol-therapy-for-chronic-gout-management-a-cost-effectiveness-analysis/