Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Mortality in Systemic Lupus Erythematosus (SLE) is increased compared to the general population. We sought to investigate mortality rates and associated factors in a cohort of refractory SLE patients treated with Rituximab (RTX).
Methods: Patients recruited to a national biologics registry (first or repeat cycle of RTX-treated and standard of care-SOC) groups were included. Demographics, concurrent medication use, disease activity (BILAG 2004/SLEDAI-2K) and damage scores (SLICC/ACR-Damage Index -SDI) were recorded. Information regarding mortality and infection was collected from study centres and the national death registry. Serious infection events (SIEs) were defined as any infection resulting in treatment with intravenous antibiotics, hospitalization, disability or death. An infection occurring with 9 months of treatment was classified as associated with RTX. Logistic regression analysis was performed using Stata (v14).
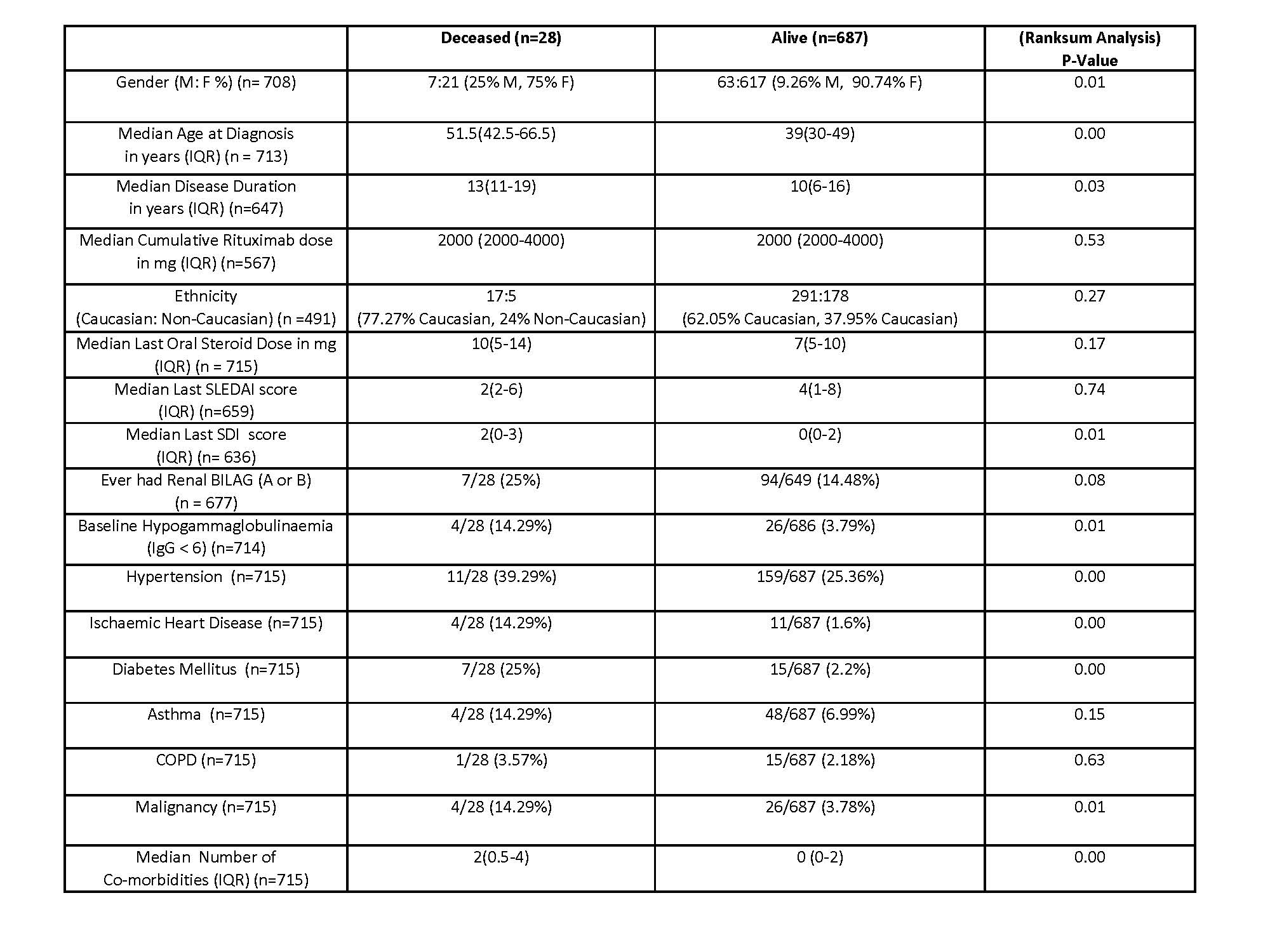
Results: 830 patients were included (715 RTX and 115 SOC-treated) with 2693 person years of follow-up. 33 deaths were reported and there was no difference in mortality between RTX and SOC groups – 28 (3.9%) RTX-treated patients died vs 5 SOC (4.3%) (1.2 deaths vs 1.5 deaths/100 pt yrs follow up). Median cumulative RTX dose did not differ between deceased and alive groups.
The primary cause of death was identifiable in 24 RTX-treated patients and included infection (n=11, 45.83%), ischaemic heart disease (n=5, 20.83%) and malignancy (n=4, 16.67%). Factors associated with mortality in the RTX group included male sex, disease duration, older age at diagnosis, hypogammaglobulinaemia and higher SDI. There was also an excess of traditional cardiovascular risk factors including hypertension and diabetes in the deceased group (Table 1).
125 SIEs occurred in 56 patients (7.8%) and 18 SIEs occurred in 7 patients (6.1%) in the RTX and SOC groups respectively during 9 months follow-up. SIE rates did not differ between groups (5.29 in RTX vs 5.46 in SOC /100 pt. yrs. follow up). The commonest SIEs in the RTX group were respiratory (n= 35, 28%). Patients who suffered SIEs within the RTX group had significantly higher baseline steroid dosing (15mg vs 10mg, p= 0.01) and demonstrated a trend to older age at recruitment, renal involvement, hypogammaglobulinaemia and higher SDI (Table 2).
Logistic regression analysis of the RTX group identified previous myocardial infarction (OR= 12.33, 95% CI 1.57-97.48), hypogammaglobulinaemia (OR= 4.25, 95% CI 1.06-16.91), diabetes (OR= 4.30, 95% CI =1.55-11.86), malignancy (OR= 3.64, 95% CI 1.51-8.81), respiratory disease (OR 2.61, 95% CI 1.15-5.90), total previous immunosuppressant use (OR 1.27, 95% CI = 1.02-1.58), SDI (OR 1.26, 95% CI 1.05-1.51) and baseline steroid use (OR 1.02, 95% CI 1.00-1.05) as significant predictors of SIEs within 9 months of treatment.
Conclusion: Despite having refractory disease, RTX-treated patients did not exhibit increased mortality or SIE rates compared to patients receiving SOC. Mortality was instead associated with co-morbidities as well as some SLE severity markers. Monitoring higher risk individuals more closely and proactive management of these risk factors may further improve outcomes in this refractory population.
To cite this abstract in AMA style:
McDonald S, McCarthy E, Aksoy A, Parker B, Bruce I. Rituximab Treatment Is Not Associated with Increased Risk of Infection or Mortality in Refractory SLE Patients: Results from the British Isles Lupus Assessment Group Biologics Registry (BILAG-BR) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/rituximab-treatment-is-not-associated-with-increased-risk-of-infection-or-mortality-in-refractory-sle-patients-results-from-the-british-isles-lupus-assessment-group-biologics-registry-bilag-br/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-treatment-is-not-associated-with-increased-risk-of-infection-or-mortality-in-refractory-sle-patients-results-from-the-british-isles-lupus-assessment-group-biologics-registry-bilag-br/