Session Information
Date: Tuesday, November 12, 2019
Title: Miscellanous Rheumatic & Inflammatory Disease Poster III: Autoimmune Conditions and Therapies
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Interstitial lung disease (ILD) is a major cause of morbidity and mortality in patients with connective tissue diseases (CTD). Approximately one-third of patients with ILD have autoimmune manifestations not classifiable as a CTD, designated as interstitial pneumonia with autoimmune features (IPAF). To our knowledge, there are no studies examining the efficacy of rituximab (RTX) in IPAF. Here, we describe a case series of 19 patients with IPAF treated with RTX.
Methods: An institution-wide registry of patients seen consecutively at a large academic medical center from 2000-2018 was queried for patients aged ≥18 years with diagnostic codes for ILD, treatment with RTX, and positive autoantibodies (including ANA, RF, anti-CCP, anti-dsDNA, anti-Ro, anti-La, anti-Smith, anti-Scl-70, anti-tRNA synthetase, anti-PM-Scl, anti-melanoma differentiation-associated protein 5). Patients included met the 2015 classification criteria for IPAF (N=19) (Fischer A, et al., Eur Respir J 2015; 46:976-87). Patients were excluded if they had received RTX for a known autoimmune disease or malignancy. Clinical data and pulmonary function tests (PFTs) were collected by medical record review. Chest computed tomography (CT) scans were reviewed independently by 2 radiologists. Clinical improvement was based on a composite of clinician assessment, oxygen requirements, hospitalization, and survival. Only those subjects with PFTs within 3 months prior to or 1 month after RTX initiation and 6-18 months after RTX initiation were included in the PFT analysis (N=10); PFT improvement was defined as ≥10% improvement in forced vital capacity (FVC).
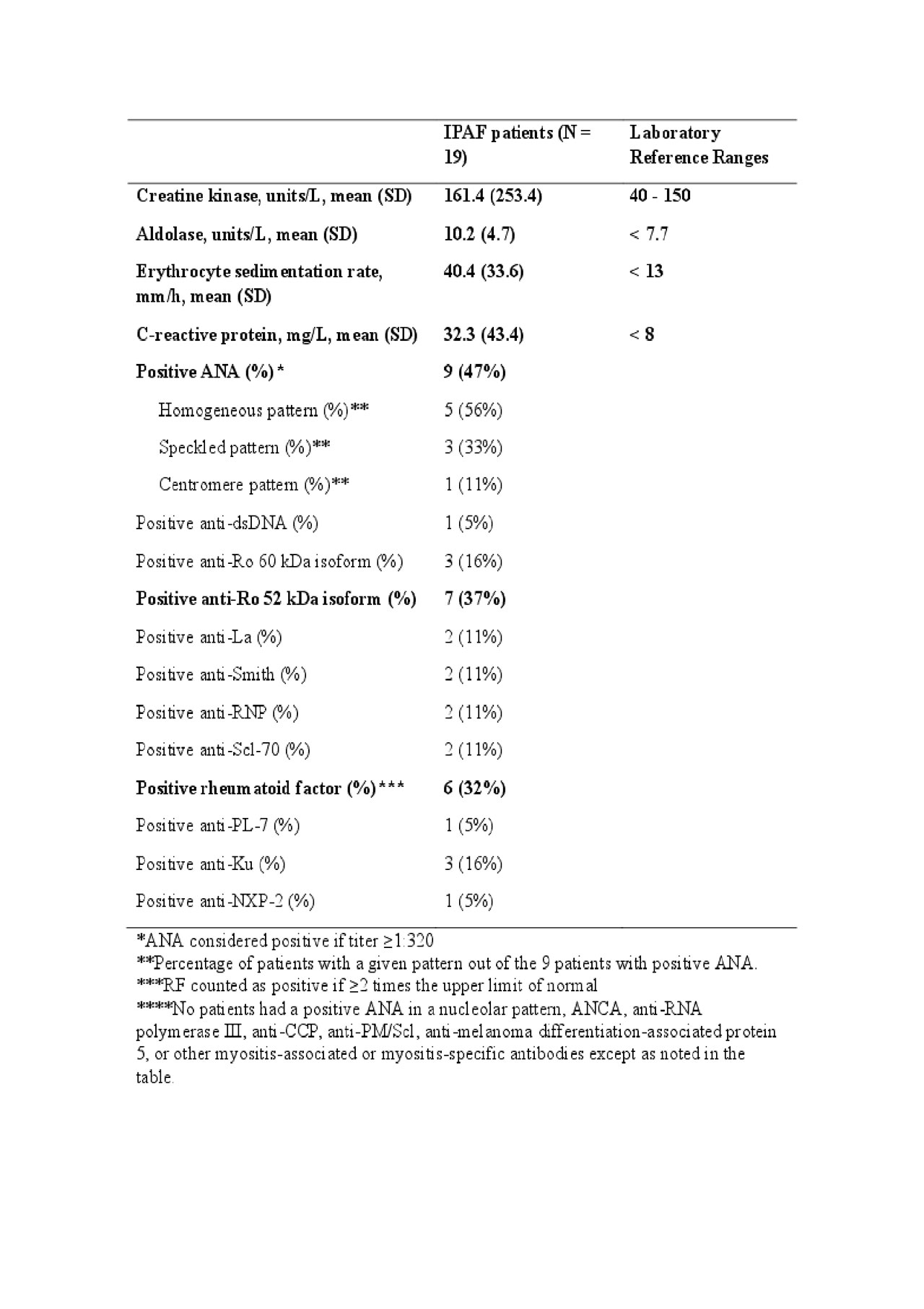
Results: To date, we have identified 19 patients with IPAF treated with RTX, with median follow up time of 24 months (Table 1). Several patients had inflammatory arthritis, myalgia, esophageal symptoms, and Raynaud phenomenon at the time of diagnosis. Sixteen patients had chest CT features of non-specific interstitial pneumonia (NSIP), organizing pneumonia (OP), or a combination of NSIP and OP. The creatine kinase, aldolase, ESR, and CRP levels were elevated (Table 2). The average number of RTX doses received was 7, over a median follow up time of 24 months (range 8-120 months).
After treatment with RTX, sixteen patients (84%) were improved or stable based on clinical parameters (Table 3). Among the ten patients with PFTs within the designated timeframe, the absolute changes in FVC percent predicted was +8% (SD 13%) and diffusion capacity of carbon monoxide (DLCO) was +3% (SD 13%). No patients stopped therapy due to adverse events. One patient died due to respiratory failure. Six patients were tapered off glucocorticoids completely after 1 year of treatment with RTX.
Conclusion: In this case series of 19 patients with IPAF treated with RTX, most patients appear to have had improvement or stability. These findings call for prospective studies, including potential randomized controlled trials, to further determine the risks and benefits of RTX use in IPAF.
To cite this abstract in AMA style:
D'Silva K, Bolster M, Castelino F, Sharma A, Little B, Montesi S, Choi H. Rituximab Therapy for Interstitial Pneumonia with Autoimmune Features (IPAF): A Case Series of Nineteen Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/rituximab-therapy-for-interstitial-pneumonia-with-autoimmune-features-ipaf-a-case-series-of-nineteen-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-therapy-for-interstitial-pneumonia-with-autoimmune-features-ipaf-a-case-series-of-nineteen-patients/