Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: More than 40 studies containing data about adults affected by EGPA treated with Rituximab (RTX) have been published in the last decade. Nevertheless, due to observational design and low methodological quality, its efficacy and safety is still unclear. We conducted a systematic review of the available clinical studies in order to examine the case for undertaking a randomized trial by providing an explicit evaluation of the weaknesses of available research and to provide findings useful to inform the design of a future trial.
Methods: MEDLINE, SCOPUS, Web of Science and Cochrane Central Register were searched up to end of January 2019 without language restriction. Reference lists of pertinent studies were analyzed through manual search to identify additional relevant studies. Missing information or data were required by email to corresponding authors. Articles and conference abstracts including at least one patient affected by EGPA, treated with RTX and assessing clinical outcomes were included and analyzed by four independent investigators and data extracted in a structured form. Following data were collected: patients’ characteristics (age, sex), sample size, diagnostic criteria used, baseline and final (at latest follow-up) vasculitis activity score, disease extension (organ involvement) and refractoriness, Rituximab schedule of administration, co-treatments, median follow-up (months), complete and partial remission rate (according to given definitions) and other secondary outcomes: steroid reduction (PDN≤7.5 mg/die), relapses, serious adverse events, infections, neoplasms and deaths.
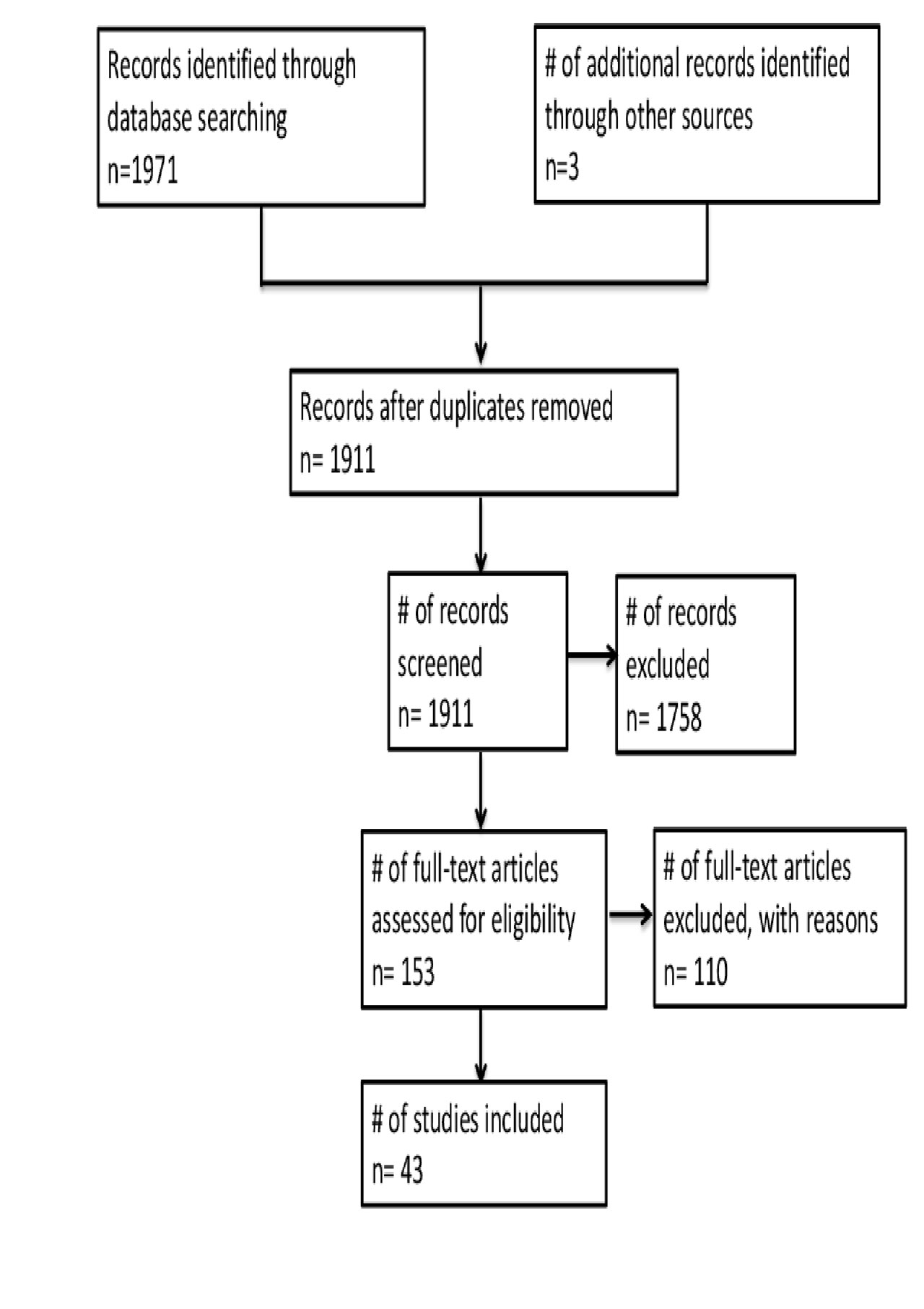
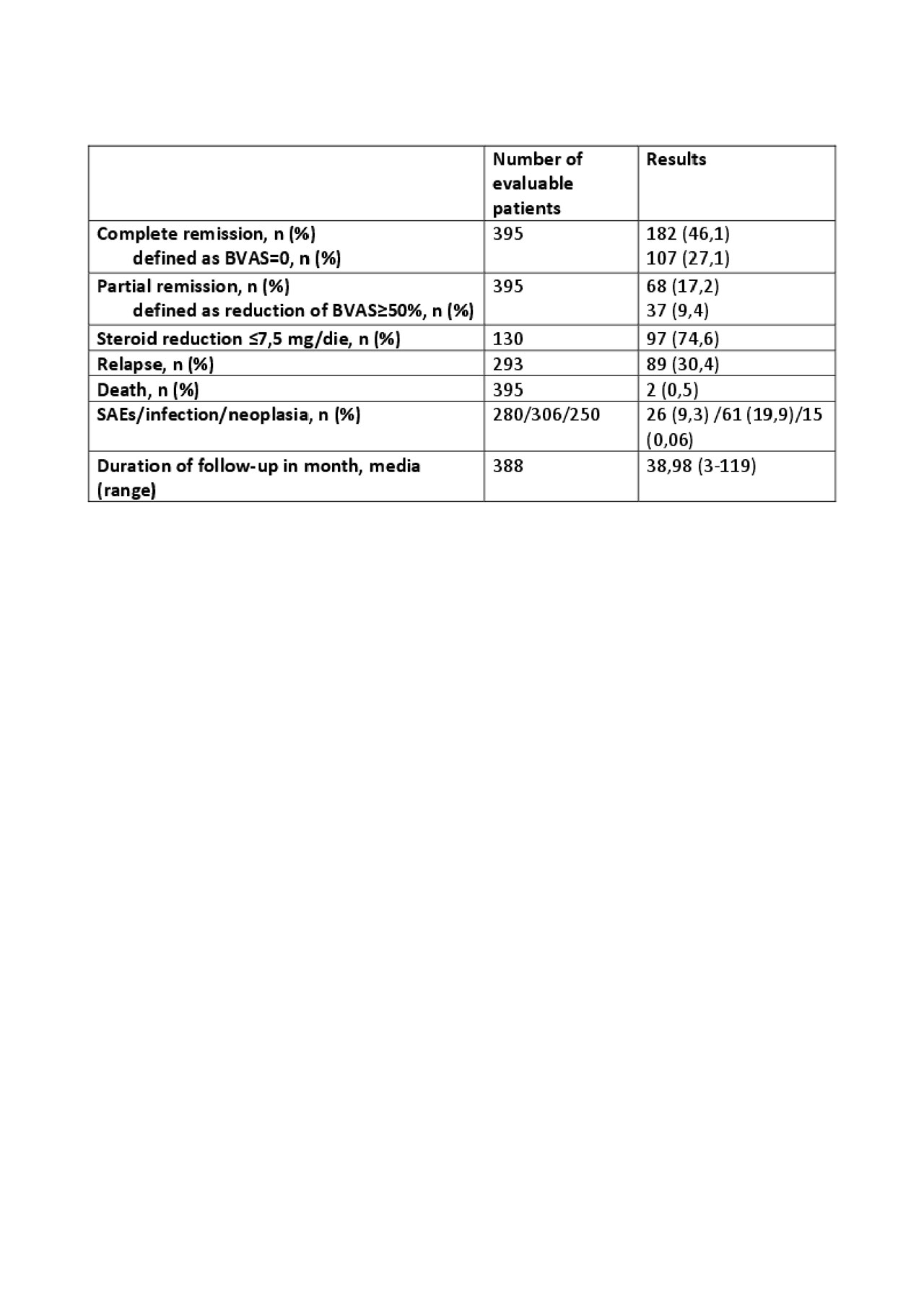
Results: A total of 1973 studies were identified, 43 of which met inclusion criteria (see figure 1), accounting for 395 EGPA patients treated with Rituximab. All of the studies were case reports, case series or retrospective cohort studies. Analysis of retrieved data is shown in table 1 and table 2. Overall, 46,1% and 17,2% patients achieved complete and partial remission respectively. A number of counfonding factors affecting the probability to reach remission has been identified and discussed. In particular, the use of standardized criteria for diagnosis and remission definition, the ‘ad hoc’ outcome selection, and the number of enrolled patients strongly influence the likelihood of response. In particular, case reports tend to overestimate efficacy of the treatment due to publication bias.
Conclusion: Rituximab might be an effective therapeutic alternative in severe refractory EGPA. However, despite high number (considering the low prevalence of the disease) of RTX treated EGPA patients described in available literature, the high risk of positive effect overestimation which tipically affects case reports and low quality observational studies, as well as flaws in outcome selection, definition and measurement hinder the possibility to achieve reliable conclusions about efficacy and safety of this therapeutic approach. Results from this systematic review could be hopefully used to better design future clinical trials to clarify this relevant topic.
To cite this abstract in AMA style:
Pomponio G, Menditto V, Rossetti G, Angeletti A, Olivari D, Gabrielli A. Rituximab for Eosinophilic Granulomatosis with Polyangiitis: A Systematic Review of Observational Studies [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/rituximab-for-eosinophilic-granulomatosis-with-polyangiitis-a-systematic-review-of-observational-studies/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-for-eosinophilic-granulomatosis-with-polyangiitis-a-systematic-review-of-observational-studies/