Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Individuals with rheumatoid arthritis (RA) have an increased risk of venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), compared with non-RA populations based on several recent studies1,2. However, information is sparse on the risk of VTE among patients receiving treatment with specific disease-modifying antirheumatic drugs (DMARDs) or categories of therapies. The objective was to estimate the incidence of VTE among patients receiving routine clinical care for RA, specifically during treatment with conventional (c) and biologic (b) DMARDs.
Methods: Incidence rates were estimated in a retrospective cohort study of patients with RA (defined as at least 2 International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9-CM] Diagnostic codes) enrolled in US health insurance plans between October 1, 2010 to September 30, 2015 and participating in the Innovation in Medical Evidence Development and Surveillance (IMEDS) program. These data are formatted into the U.S. Food and Drug Administration’s Sentinel Common Data Model and Sentinel’s publicly available standardized analysis tools were used to estimate the incidence rates of VTE following initiation of cDMARDs or bDMARDs. Patients were required to be new users of the study drug class, with no evidence of use in the 365 days preceding initiation and were not allowed to re-enter the cohort. Patients were required to demonstrate continuous use of the study drug class to be considered at risk for VTE. VTE was defined based on ICD-9-CM codes, but patients diagnosed in outpatient settings were also required to have evidence of oral anticoagulant dispensing within 31 days of the event. PE and DVT ICD-9-CM codes were also disaggregated to produce separate incidence rates.
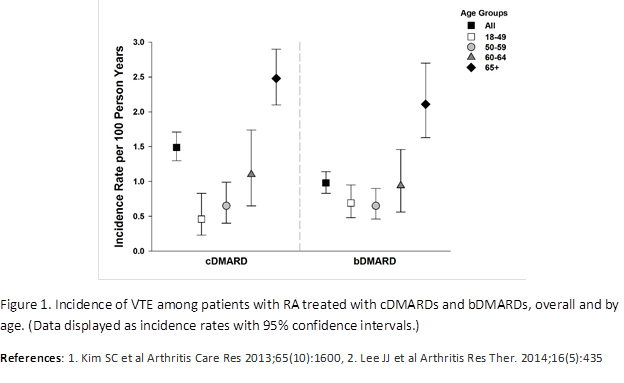
Results: During treatment with any cDMARDs (methotrexate or leflunomide) or bDMARDs (abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, and tocilizumab), patients experienced VTE at a crude incidence rate of 1.49 (95% CI 1.30, 1.71) per 100 person-years (PY) and 0.98 (95% CI 0.83, 1.14) per 100 PY, respectively. For each study drug class, age was an important risk factor for VTE, with increasing age associated with higher rates of VTE; for example, during treatment with bDMARDs incidence rate (IR)18-49 years=0.69, IR50-59 years=0.65, IR60-64 years=0.94, and IR65+ years=2.11 per 100 PY (Figure 1). Men also had higher incidence rates of VTE than women. In the analyses of PE and DVT, DVT incidence rates were higher than PE incidence rates, and similar incidence rate trends overall and across age strata for cDMARD and bDMARD cohorts were noted.
Conclusion: Venous thromboembolism is an important clinical concern among patients with RA and incidence rates vary by age and sex during routine clinical treatment with DMARDs.
To cite this abstract in AMA style:
Maro J, Menzin T, Hornbuckle K, Giles JT, Kavanaugh A, Dörner T, Martin D, Huang J, Salinas CA. Risk of Venous Thromboembolism in Rheumatoid Arthritis Patients Treated with Biologic and Non-Biologic Dmards [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/risk-of-venous-thromboembolism-in-rheumatoid-arthritis-patients-treated-with-biologic-and-non-biologic-dmards/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-venous-thromboembolism-in-rheumatoid-arthritis-patients-treated-with-biologic-and-non-biologic-dmards/