Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Importance
The efficacy of Janus kinase (JAK) inhibitors is well established across a range of diseases. However, there is a major concern regarding the potential risk of an increased number of thromboembolic adverse events.
Objective
To assess the risk of thromboembolism in patients treated with JAK inhibitors.
Methods: Data Sources
Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus were searched (Inception to May 23, 2019).
Study Selection
Published randomized, placebo-controlled trials (Phase II and III) that evaluated JAK inhibitor therapies in any disease and had reported safety data.
Data Extraction and Synthesis
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Two investigators independently extracted study data, and assessed risk of bias.
Main Outcomes and Measures
The outcome of interest was the number of thromboembolic events in individuals receiving JAK inhibitor therapies compared to placebo. We used the Peto fixed effects model to calculate the pooled odds ratios as this is the appropriate model for rare events.
Results: Results
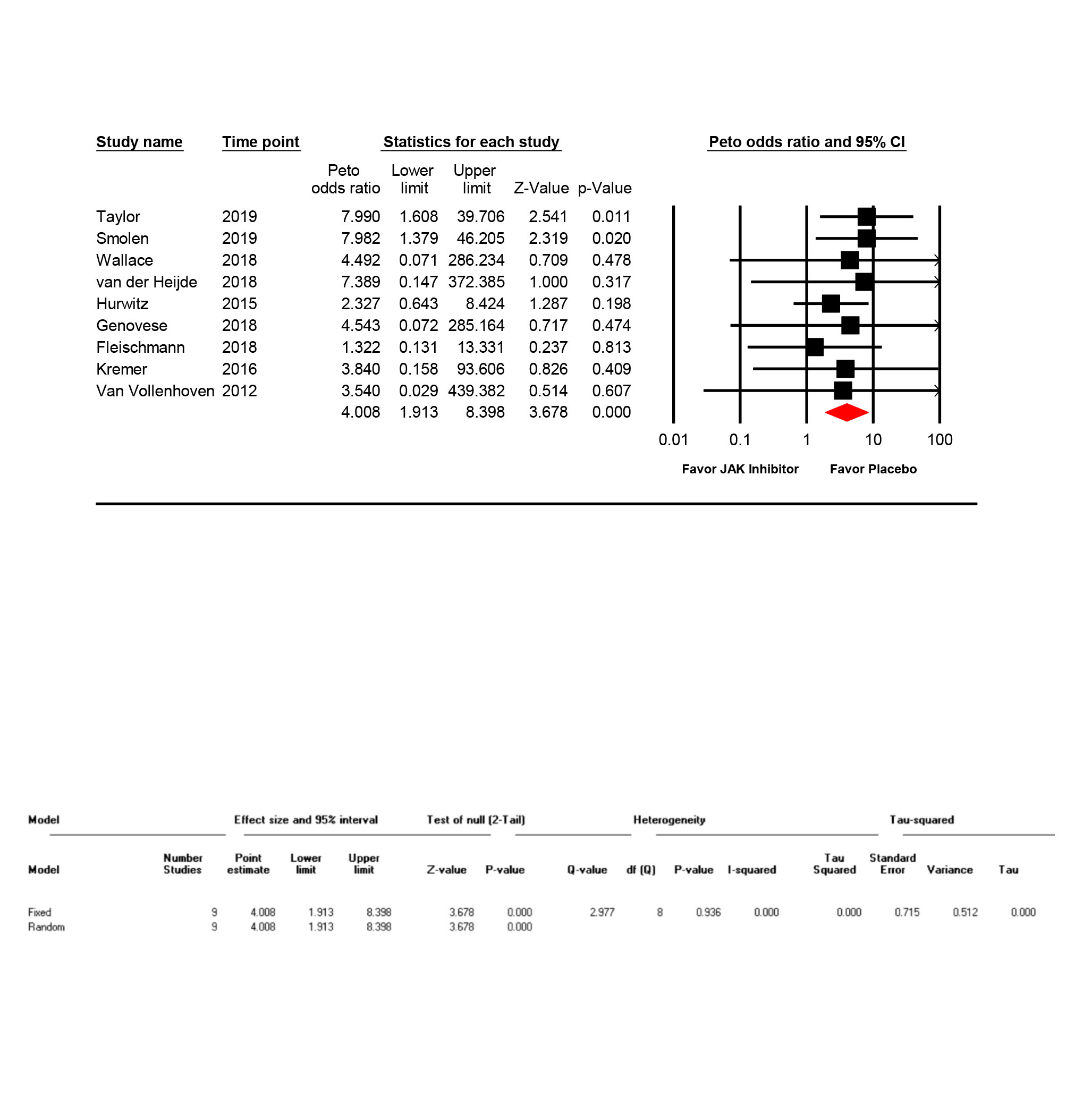
We included 9 eligible RCTs (9496 patients). Patients receiving JAK inhibitors had higher risk of thromboembolic events OR 4.09 (95% CI 1.91-8.48, P < 0.001, I2=0%;)(figure 1). The results remained consistent with continuity correction OR 3.56 (95% CI 1.74-7.30, P=0.001, I2=0%), and after excluding studies with cancer patients OR 2.98 (95% CI 1.48-6.00, P=0.002, I2=0%). Medications wise analysis showed OR 7.65 (95% CI 2.45-23.88, P=0.002, I2=0%) with bariticinib (3 trials), OR 1.75 (95% CI 0.74-4.13, P=0.19, I2=0%) with ruxolitinib (2 trials), and OR 2.21 (95% CI 0.40-12.17, P=0.36, I2=0%) with upadacitinib (3 trials). There were 2 trials of tofacitinib and outcome of interest was reported in one trial only which is why drug wise analysis of tofacitinib was not performed.
Limitations
Short follow up duration
Conclusion: Conclusions
Risk of thromboembolism is increased in patients who are treated with JAK inhibitors as compared to placebo. The findings remained consistent even when studies with cancer patients were excluded. In subgroup analyses based on individual medications, only bariticinib showed statistically significant findings of increased thrombosis risk. This analysis provides toxicity estimates for thromboembolic events associated with the use of JAK inhibitors that can inform shared-decision making when patients and clinicians are contemplating the use of JAK inhibitors for various indications.
To cite this abstract in AMA style:
Bilal J, Riaz I, Sadiq M, Salick M, Nomaan Y, Iqbal N, Bhattacharjee S, Prokop L, Kwoh C. Risk of Thromboembolism with Janus Kinase Inhibitors: A Systematic Review and Meta-Analysis of Randomized Placebo Controlled Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-of-thromboembolism-with-janus-kinase-inhibitors-a-systematic-review-and-meta-analysis-of-randomized-placebo-controlled-trials/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-thromboembolism-with-janus-kinase-inhibitors-a-systematic-review-and-meta-analysis-of-randomized-placebo-controlled-trials/