Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: It is well-known that biologic or targeted synthetic DMARDs increase the risk of serious infections (SIs), but few studies have directly compared the risk of SI across these DMARDs in a real-world setting. We aimed to compare incidence rate (IR) of serious bacterial, viral or opportunistic infection in rheumatoid arthritis (RA) patients initiating tofacitinib (TOF) (common exposure) versus other biologic DMARDs: abatacept (ABA), adalimumab (ADA), certolizumab (CER), etanercept (ETA), golimumab (GOL), infliximab (INF) or tocilizumab (TCZ).
Methods: We analyzed data from 3 U.S. healthcare claims databases: Medicare (2012-2015), Optum (2012-09/2017) and MarketScan (2012-2017). RA patients aged≥18 years who initiated TOF or other biologic DMARD without any prior use of biologics or JAK inhibitors were identified. We excluded patients with recent infection, malignancy or rituximab use. The primary outcome was a composite endpoint of incident SI that included bacterial, viral or opportunistic infection based on the inpatient principal diagnosis code. Secondary outcomes were specific subtypes of SIs. We adjusted for >70 potential confounders including demographics, prior DMARD and antibiotic use, comorbidities, other medications, and healthcare utilization factors in each database through propensity score (PS)-based inverse probability treatment weighting. For the as-treated analysis, follow-up time started the day after cohort entry until the earliest of: treatment discontinuation, switching to any biologic other than index therapy, nursing home admission, death, disenrollment, or the end of study period. For each drug-comparison, weighted Cox proportional hazards models estimated the HRs and 95% CIs. The estimates from 3 databases were combined using an inverse variance-weighted, fixed-effects meta-analysis.
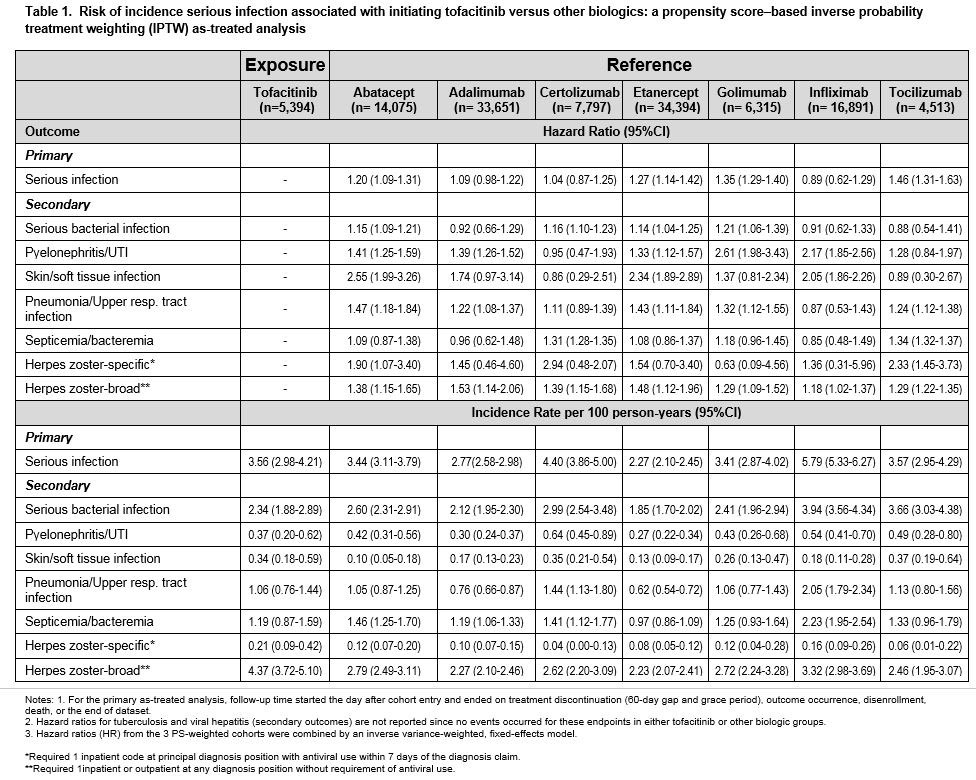
Results: A total of 123,960 biologic initiators were identified across 3 databases, of which 5,531 (4.5%) were TOF initiators. Mean age was 72 years in Medicare, 54 in Optum and 52 in MarketScan. During the 180-day baseline period, 64-71% patients used methotrexate and 68-73% used corticosteroids. After PS-weighting, all covariates were well balanced. The median follow-up time (days) in the as-treated analysis ranged from 164 (Optum) to 182 (MarketScan). A total of 2,958 SI events occurred. In the TOF group, the crude IR for SIs per 100 person-years ranged from 2.80 (MarketScan) to 7.89 (Medicare). Adjusted HRs showed higher risk of composite SIs in TOF compared to ABA (HR 1.20, 95% CI 1.09-1.31), ETA (1.27, 1.14-1.42), GOL (1.35, 1.29-1.40) and TCZ (1.46, 1.31-1.63), but similar risk compared to ADA, CER and INF. Serious bacterial infection risk was higher in TOF than ABA, CER, ETA and GOL. Secondary analyses showed consistent findings (Table 1).
Conclusion: This large multi-database cohort study of RA patients found a higher risk for the composite endpoint of SIs requiring hospitalization after initiating TOF versus ABA, ETA, GOL and TCZ as their first biologic or targeted synthetic DMARD therapy. The risk of serious bacterial infection, pneumonia, herpes zoster, and skin and soft tissue infections was also higher in TOF initiators versus several other biologics.
To cite this abstract in AMA style:
Pawar A, Desai R, Gautam N, Kim S. Risk of Serious Infections in Tofacitinib versus Other Biologic Drug Initiators in Patients with Rheumatoid Arthritis: A Multi-database Cohort Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-of-serious-infections-in-tofacitinib-versus-other-biologic-drug-initiators-in-patients-with-rheumatoid-arthritis-a-multi-database-cohort-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-serious-infections-in-tofacitinib-versus-other-biologic-drug-initiators-in-patients-with-rheumatoid-arthritis-a-multi-database-cohort-study/