Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: A variety of biologic and targeted synthetic disease modifying antirheumatic agents have been widely used among patients with psoriatic arthritis (PsA). Due to the lack of head-to-head comparison trials, the comparative risk of respiratory tract infections (RTIs) among these available agents remains unclear, however. Consequently, a network meta-analysis (NMA) was used to address this knowledge gap, and we sought to investigate the comparative risk of RTIs with different biologic and targeted synthetic disease modifying antirheumatic agents in PsA.
Methods: Medline, PubMed, Embase, Scopus, Cochrane Central, and clinicaltrials.gov were searched to identify phase 3 and 4 randomized clinical trials (RCTs) that reported safety outcomes of biologic and targeted synthetic disease modifying antirheumatic agents in PsA. Outcome of interest was occurrence of respiratory tract infection(s) within on-treatment or placebo-controlled duration. Mixed treatment comparisons were computed using NMA within the frequentist framework. Fixed effect models were used due to sparse direct evidence and open network. Effect estimates were expressed as odds ratio (OR) with their 95% confidence intervals (CIs). P-scores were calculated to establish relative rankings of different treatment options. A sensitivity analysis was conducted using Bayesian NMA approach. All statistical analyses were conducted in R (v 4.0.2).
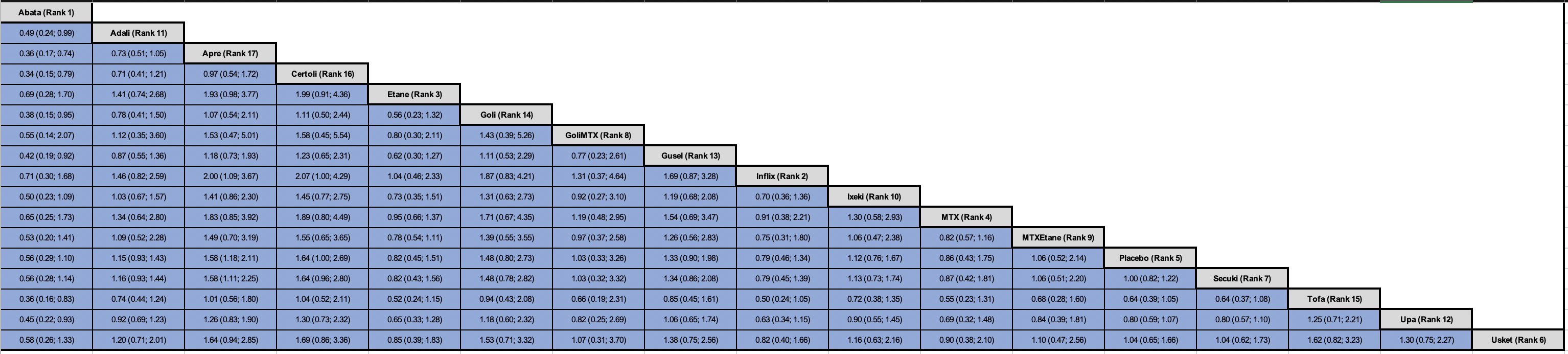
Results: A total of 33 RCTs were included in this systematic review. A total of 32 RCTs with 14,974 patients, and 17 unique treatment arms were included in the NMA and one RCT did not report any RTI events was excluded [see Figure 1.] Mixed treatment comparisons showed that abatacept (P-score 0.92) ranked as the best (lowest likelihood of RTIs) treatment compared to adalimumab (OR 0.49; 95% CI 0.24-0.99), apremilast (OR 0.36; 95% CI 0.17-0.74), certolizumab (OR 0.34; 95% CI 0.15-0.79), golimumab (OR 0.38; 95% CI 0.15-0.95), guselkumab (OR 0.42; 95% CI 0.19-0.92), tofacitinib (OR 0.36; 95% CI 0.16-0.83), and upadacitinib (OR 0.45; 95% CI 0.22-0.93). Apremilast ranked the lowest with increased likelihood of RTIs compared to infliximab (OR 2.00; 95% CI 1.09-3.67), and secukinumab (OR 1.58; 95% CI 1.11-2.25). However, no other significant differences were observed across different biologic and targeted synthetic disease modifying antirheumatic agents [Figure 2]. The results were consistent with Bayesian NMA.
Conclusion: Abatacept may have a lower risk of RTIs when used for treatment of PsA. Further analyses are warranted to assess the severity and type of RTIs associated with these treatments.
To cite this abstract in AMA style:
Liu Y, Bilal J, Ajmal M, Naqvi S, Shahid Z, Khakwani K, Gondal F, Riaz I, Bhattacharjee S, Bogucka R, Kwoh C. Risk of Respiratory Tract Infections with Biologic and Targeted Synthetic Antirheumatic Agents in Psoriatic Arthritis: A Systematic Review and Network Meta-analysis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/risk-of-respiratory-tract-infections-with-biologic-and-targeted-synthetic-antirheumatic-agents-in-psoriatic-arthritis-a-systematic-review-and-network-meta-analysis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-respiratory-tract-infections-with-biologic-and-targeted-synthetic-antirheumatic-agents-in-psoriatic-arthritis-a-systematic-review-and-network-meta-analysis/