Session Information
Date: Tuesday, November 12, 2019
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III: Giant Cell Arteritis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Oral glucocorticoids (OGC) have been the mainstay of treatment for giant cell arteritis (GCA). However, OGCs are associated with several adverse events (AEs). The purpose of this study was to estimate the risk of potential OGC-related AEs in patients with GCA.
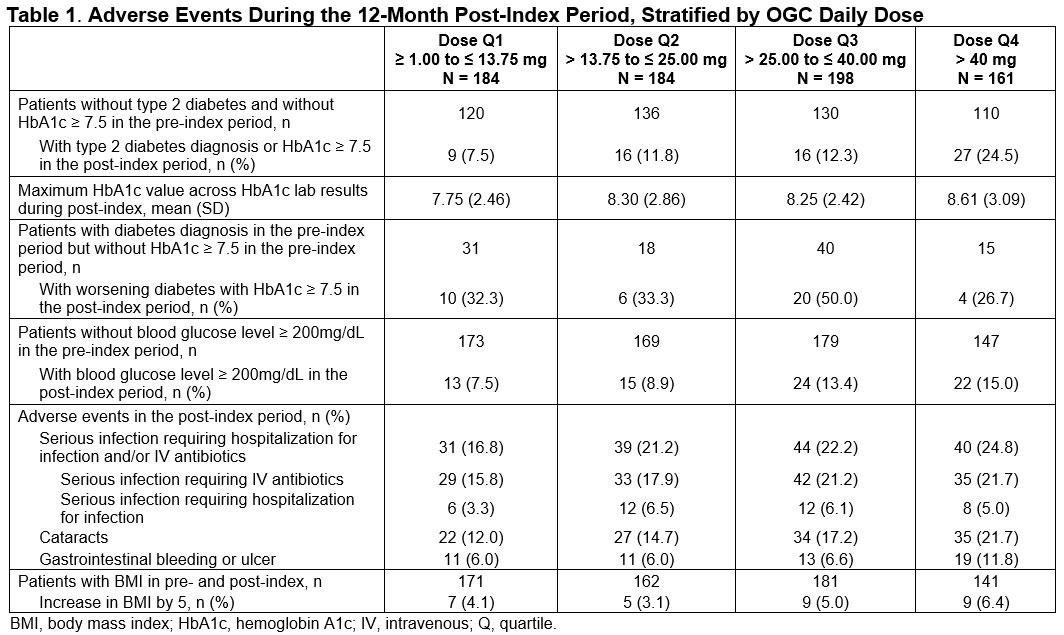
Methods: This retrospective, observational cohort study utilized the 2008-2017 IBM Explorys Electronic Health Records database which includes laboratory values. Inclusion criteria included age ≥ 50 years with ≥ 2 GCA diagnoses ≥ 7 days apart, 1 OGC prescription within 6 months of the first GCA diagnosis (index date = date of first OGC prescription) followed by a second OGC prescription, no other autoimmune disease requiring high-dose OGCs, no exposure to anti-tumor necrosis factor or anti–interleukin-6 therapies, ≥ 1 C-reactive protein (CRP)/erythrocyte sedimentation rate (ESR) laboratory test and 12 months of data available pre- and post-index. Potential AEs assessed during the 12 months post-index were descriptively summarized across cohorts of patients based on quartiles (Q) of mean daily dose of OGCs measured over 6 months post-index among this patient sample (Q1: ≥ 1.00 to ≤ 13.75 mg; Q2: > 13.75 to ≤ 25.00 mg; Q3: > 25.00 to ≤ 40.00 mg; Q4: > 40.00 mg). Potential AEs included type 2 diabetes (T2D) diagnosis, hemoglobin A1c (HbA1c), blood glucose level, serious infections, cataracts, gastrointestinal bleeding or ulcer and increases in body mass index (BMI). Actual OGC use by patient could not be confirmed and is a limitation of this study.
Results: Mean age of the 785 eligible patients was 76 years (SD 9); 70% were female. Mean Deyo Charlson Comorbidity Index score at baseline was 1.57 (SD 2.01). The most common baseline comorbid conditions were cerebrovascular disease, diabetes, chronic pulmonary disease, and renal disease. Mean daily OGC dose was 28.9 mg during the first 6 months post-index. Mean (SD) CRP and ESR during the 12-month follow-up was 5.1 (13.6) and 26.5 (20.7), respectively. The proportion of patients with newly diagnosed T2D or with HbA1c ≥ 7.5 during the 12-month follow-up ranged from 7.5% to 24.5% from OGC daily dose Q1 to Q4 cohorts. The proportion of patients with glucose ≥ 200 mg/dL ranged from 7.5% to 15.0% from Q1 to Q4. Serious infections ranged from 16.8% to 24.8% from Q1 to Q4 and cataract ranged from 12.0% to 21.7% from Q1 to Q4. The proportions of patients with gastrointestinal bleed/ulcer ranged from 6.0% in Q1 to 11.8% in Q4. An increase in BMI of 5 ranged from 4.1% to 6.4% from Q1 to Q4.
Conclusion: In patients with GCA, potential OGC-related AEs increased with increased daily OGC dose. This highlights the need for effective therapies for GCA that reduce the exposure and potential risk of OGCs.
Acknowledgements: This study was funded by Genentech, Inc.
To cite this abstract in AMA style:
Best J, Kong A, Tran O, Michalska M. Risk of Potential Glucocorticoid-Related Adverse Events in Patients with Giant Cell Arteritis: Results from a US-based Electronic Health Records Database [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-of-potential-glucocorticoid-related-adverse-events-in-patients-with-giant-cell-arteritis-results-from-a-us-based-electronic-health-records-database/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-potential-glucocorticoid-related-adverse-events-in-patients-with-giant-cell-arteritis-results-from-a-us-based-electronic-health-records-database/