Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic, progressive inflammatory arthritis that significantly impairs quality of life and contributes to substantial morbidity. Interleukin-17 (IL-17) plays a central role in PsA pathogenesis. Although anti–IL-17 therapies have shown clinical efficacy in PsA, clinical trials have reported cases of new-onset inflammatory bowel disease (IBD) or exacerbation of pre-existing IBD. Given these concerns, this study aimed to investigate whether treatment with IL-17 inhibitors is associated with a higher risk of IBD in a large real-world cohort of PsA patients, compared with other treatment options.
Methods: This was a retrospective observational analysis utilizing the TriNetX research network, which includes data from over 130 million patients globally. The study population consisted of 11,287 patients with psoriatic arthritis (PsA) treated with IL-17 inhibitors and 51,328 PsA patients receiving other therapies, such as other biologic DMARDs (bDMARDs), conventional synthetic DMARDs (csDMARDs), targeted synthetic DMARDs (tsDMARDs), or NSAIDs. Outcomes were evaluated both before and after propensity score matching for demographic and clinical factors. The risks of developing Crohn’s disease, ulcerative colitis, and other or unspecified noninfective gastroenteritis and colitis were assessed between January 1, 2015, and April 1, 2025 (Appendix 1).
Results: After propensity score matching, 8 931 patients were included in each group. The overall cohort’s mean ± SD age was 51 ± 12.9 years, with 57.586% female and the majority White (75.21%) and not Hispanic or Latino (72.064%). Matching achieved balance across cohorts (P < 0.05) (Table 1, Table 2). The IL-17 inhibitor–treated cohort demonstrated a slightly lower risk of developing Crohn’s disease compared with the non–IL-17i cohort (Relative risk (RR) 0.723, 95% CI: 0.524 – 0.998, p = 0.0475). In contrast, the IL-17i group showed a significantly lower risk of developing ulcerative colitis (RR 0.562, 95% CI: 0.414– 0.763, p < 0.01) and other or unspecified noninfective gastroenteritis and colitis (RR 0.761, 95% CI: 0.673– 0.861, p < 0.01).
Conclusion: This study suggests that treatment with IL-17 inhibitors in patients with psoriatic arthritis does not appear to increase the risk of developing or exacerbating Crohn’s disease. Additionally, IL-17 inhibitors may be associated with a decreased risk of developing ulcerative colitis and other unspecified noninfective gastroenteritis and colitis compared to other treatment options. Further research is warranted to better understand the mechanisms underlying these observations.
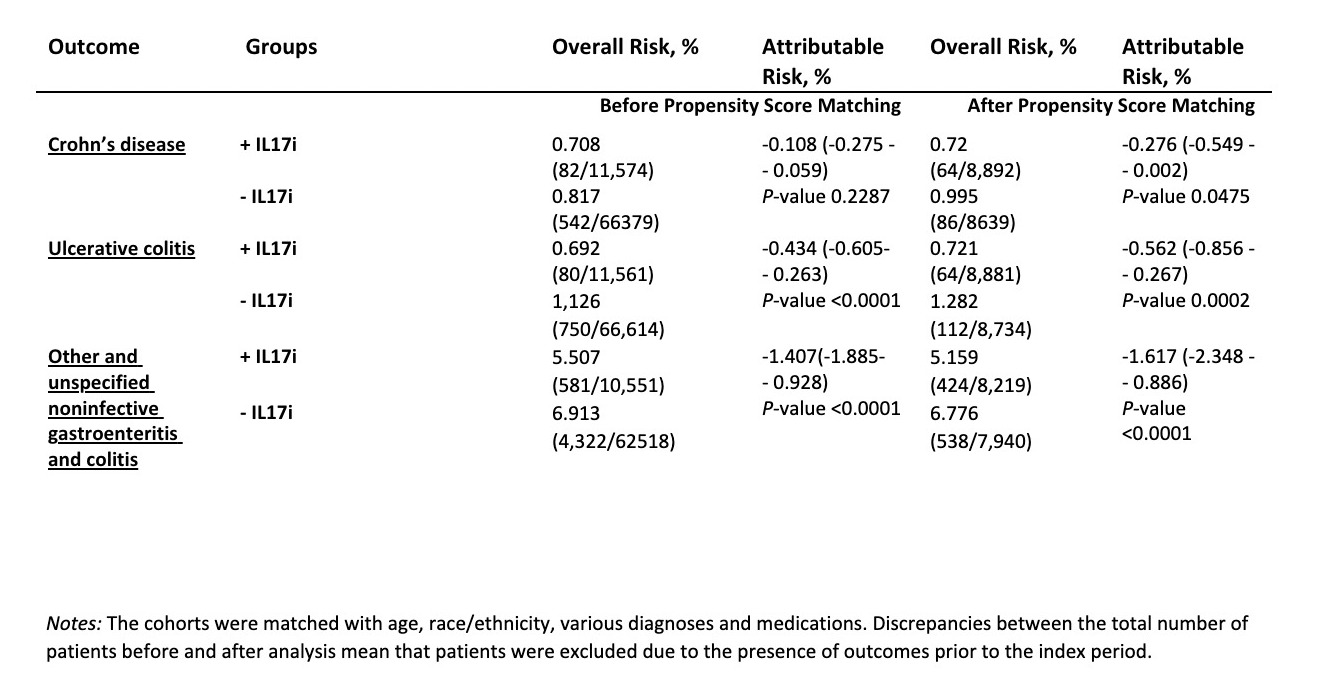 Table 1. Baseline Characteristics of Study Groups Before and After Propensity Score Matching
Table 1. Baseline Characteristics of Study Groups Before and After Propensity Score Matching
.jpg) Table 2. Risks of Crohn’s Disease, Ulcerative Colitis, and Other or Unspecified Noninfective Gastroenteritis and Colitis in Psoriatic Arthritis Patients With vs Without IL-17i Exposure
Table 2. Risks of Crohn’s Disease, Ulcerative Colitis, and Other or Unspecified Noninfective Gastroenteritis and Colitis in Psoriatic Arthritis Patients With vs Without IL-17i Exposure
.jpg) Appendix 1. Inclusion and Exclusion Criteria, Matching Criteria and Outcomes with Corresponding ICD-10 and RxNorm Codes
Appendix 1. Inclusion and Exclusion Criteria, Matching Criteria and Outcomes with Corresponding ICD-10 and RxNorm Codes
To cite this abstract in AMA style:
Tskitishvili R, Hussein A, Tskhakaia I, Tsibadze N, Osgood E, Khan H. Risk of Inflammatory Bowel Disease in Psoriatic Arthritis Patients Treated with IL-17 Inhibitors [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-of-inflammatory-bowel-disease-in-psoriatic-arthritis-patients-treated-with-il-17-inhibitors/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-inflammatory-bowel-disease-in-psoriatic-arthritis-patients-treated-with-il-17-inhibitors/
