Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders II: Clinical
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Exposure to the interleukin-6 inhibitor tocilizumab has been associated with an increased risk of gastrointestinal (GI) perforation in patients with rheumatoid arthritis. No studies to date have evaluated the risk of GI perforation in patients with giant cell arteritis (GCA) on treatment with tocilizumab. This study aimed to describe the incidence and risk factors associated with GI perforation among incident cases of GCA receiving treatment with tocilizumab.
Methods: We performed a retrospective cohort study of incident cases of GCA using the US-based TriNetX electronic health records database from 1/1/2010 to 4/23/2023. Patients were included if they had (1) 2 ICD-9CM/ICD10-CM codes for GCA separated by 30 days but within 1 year and (2) received any dose of prednisone within 30 days of the first GCA code. Gastrointestinal perforations were defined by ICD-9-CM/ICD-10-CM codes as described previously, and the incidence of gastrointestinal perforations as well as unadjusted incident rate ratios were calculated.1 Adjusted analysis using a Poisson regression was conducted. The clone-censor-weight approach was then used to account for immortal time bias. After cloning, censoring, and weighting using inverse probability of censoring, time-updated multivariable Cox proportional hazards models were used to calculate the hazard ratio (HR) and 95% confidence intervals for the risk of GI perforation.
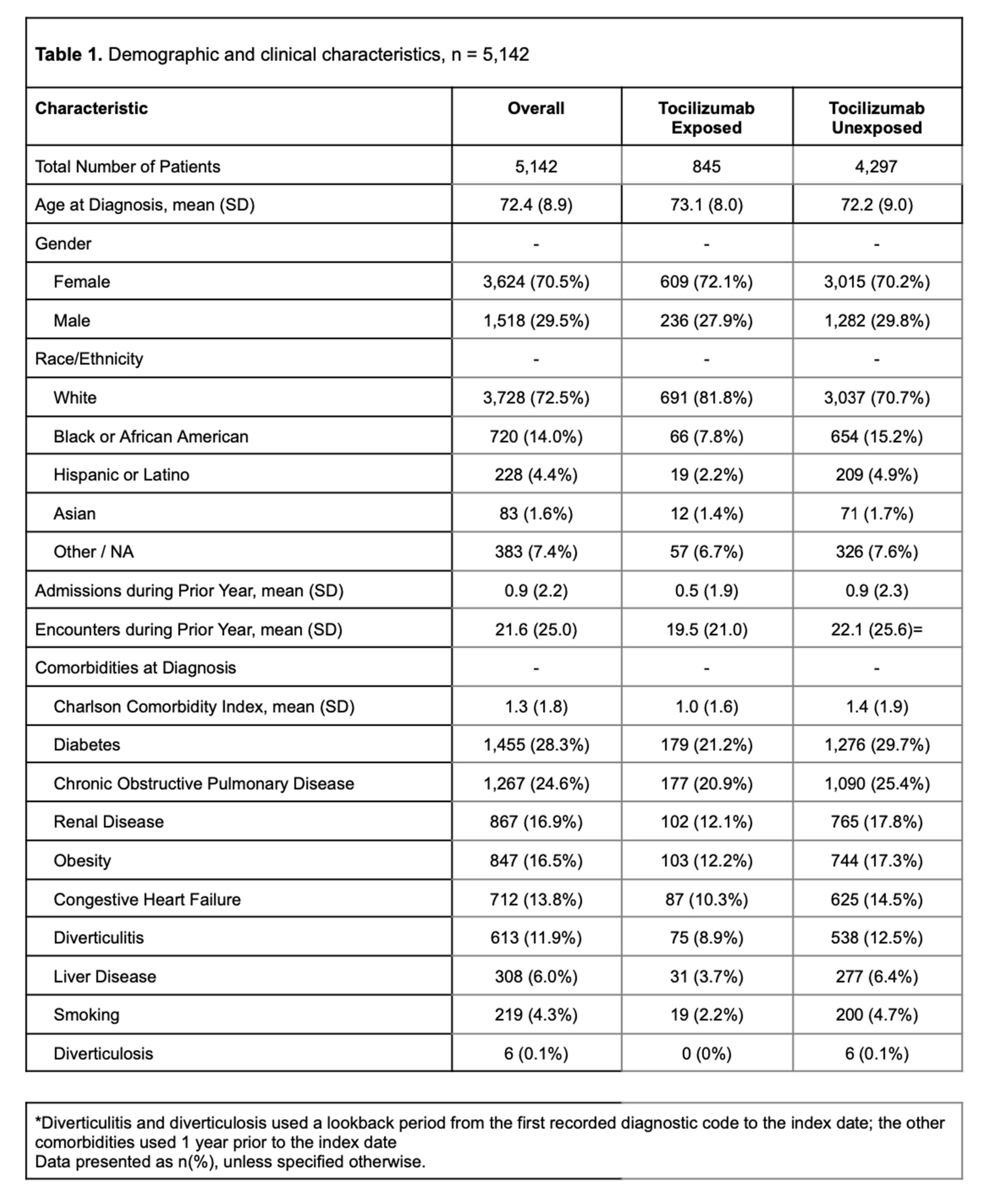
Results: During the study period, 5,142 patients met the inclusion criteria (845 tocilizumab exposed and 4,297 tocilizumab unexposed), the majority of whom were female (3,624, 70.5%) and white (3,728, 72.5%) (Table 1). Incident GI perforations among tocilizumab exposed vs. unexposed were 2.0/1,000 person-years and 3.4/1,000 person-years, respectively, resulting in an incident rate ratio of 0.57 (95% CI 0.14-2.41). The adjusted rate ratio (RR) of GI perforation with tocilizumab use in a Poisson regression was RR 0.56 (95% CI 0.13-2.38). Factors associated with GI perforation included the history of diverticulitis (RR 3.51, 95% CI 1.55-7.96) and the use of intravenous methylprednisolone (RR 5.41, 95% CI 2.41-12.12). After implementing the clone-censor-weight approach, tocilizumab exposure was not associated with an increased risk of GI perforation (HR 1.05, 95% CI 0.30-1.65).
Conclusion: In this retrospective cohort study of patients with incident GCA, GI perforations were rare. When compared with steroid treatment, tocilizumab exposure was not associated with an increased risk of GI perforation. Risk factors for GI perforation included a history of diverticulitis and intravenous methylprednisolone use. These findings highlight the importance of judicious steroid use and will be useful for counseling patients considering initiation of IL-6 inhibitors.
Reference
1. Curtis JR. Arthritis Rheum. 2011 Feb;63(2):346
To cite this abstract in AMA style:
Nepal D, Sattui S, Wallace Z, Putman M. Risk of Gastrointestinal Perforation Among Patients with Giant Cell Arteritis Who Received Tocilizumab [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/risk-of-gastrointestinal-perforation-among-patients-with-giant-cell-arteritis-who-received-tocilizumab/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-gastrointestinal-perforation-among-patients-with-giant-cell-arteritis-who-received-tocilizumab/