Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Counselling patients with rheumatic and musculoskeletal diseases (RMD) in the phase of reproduction, pregnancy and breastfeeding is a challenging task. The potential risk of drug therapy must be weighed against the risk of untreated RMD for the patient and the risk of fertility issues and unfavourable pregnancy outcomes. Therefore, inactive disease is the treatment target and the prerequisite for improved outcomes. Given the limited amount of high quality data on many anti-rheumatic drugs, we analysed the extent to which drug recommendations in daily practice may be related to considerations of disease activity.
Methods: The current EULAR task force on antirheumatic drugs in reproduction, pregnancy and lactation includes 21 international experts prescribing drugs. In December 2023 these experts were asked to fill in a web-based questionnaire similar to the one used for the 2016 EULAR points to consider for use of antirheumatic drugs in pregnancy and breastfeeding [1]. The questionnaire addressed drug use before or during pregnancy, during breastfeeding and in male patients planning to conceive. For each drug, the expert had to choose one of the four options: (1) recommend the drug in the same way as if the patient was not pregnant / breastfeeding / planning to conceive, (2) only recommend the drug if one feared at least moderate or (3) severe disease in its absence, or (4) would never recommend the drug in pregnancy / while breastfeeding / in a male patient planning to conceive.
[1] Gotestam Skorpen C et al. Ann Rheum Dis. 2016 May;75(5):795-810.
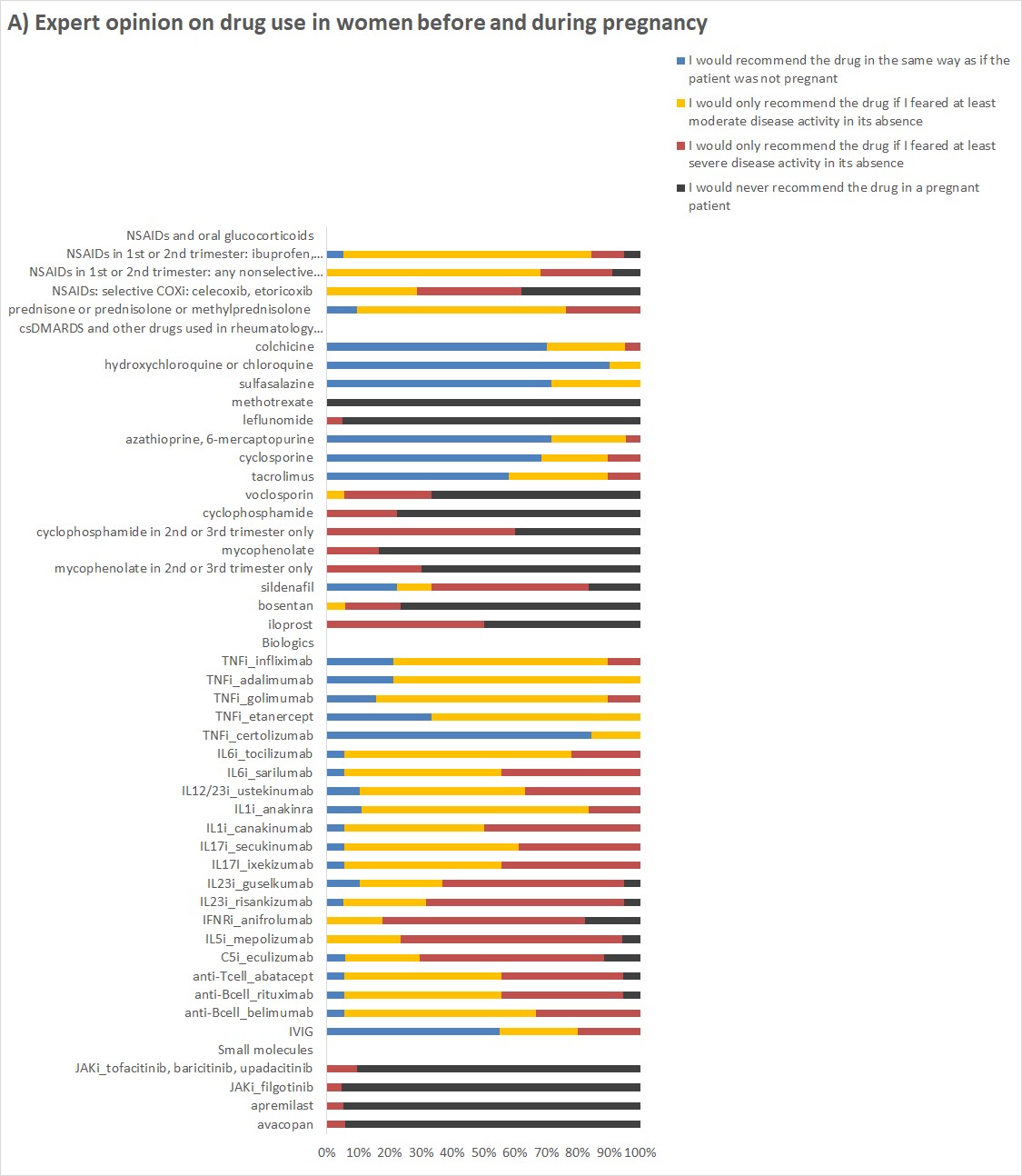
Results: The expert opinion on the use of drugs in women before and during pregnancy (Figure 1A) showed that the majority recommended pregnancy-compatible csDMARDs, the TNFi certolizumab and IVIG in the same way as if the patient was not pregnant. The use of other TNFi and non-TNFi biologics in pregnant women was primarily recommended if there was a risk of moderate or severe disease activity without the drug. Similarly, experts recommended NSAIDs and oral glucocorticoids almost exclusively for the control of active disease states.
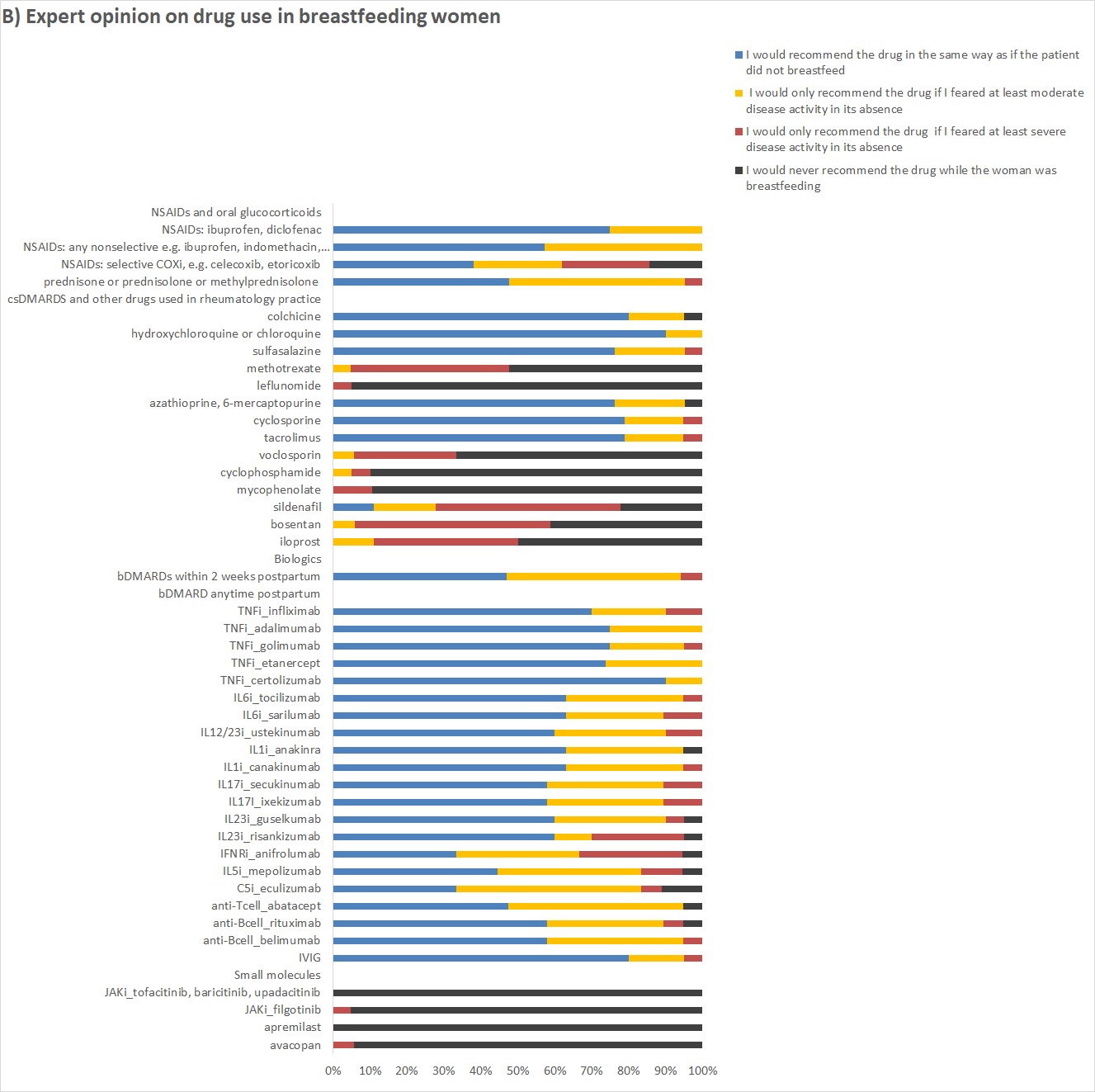
In breastfeeding women TNFi and other biologics, compatible csDMARDs, NSAIDs and oral glucocorticoids were most often recommended in the same way as if the patient did not breastfeed (Figure 1B).
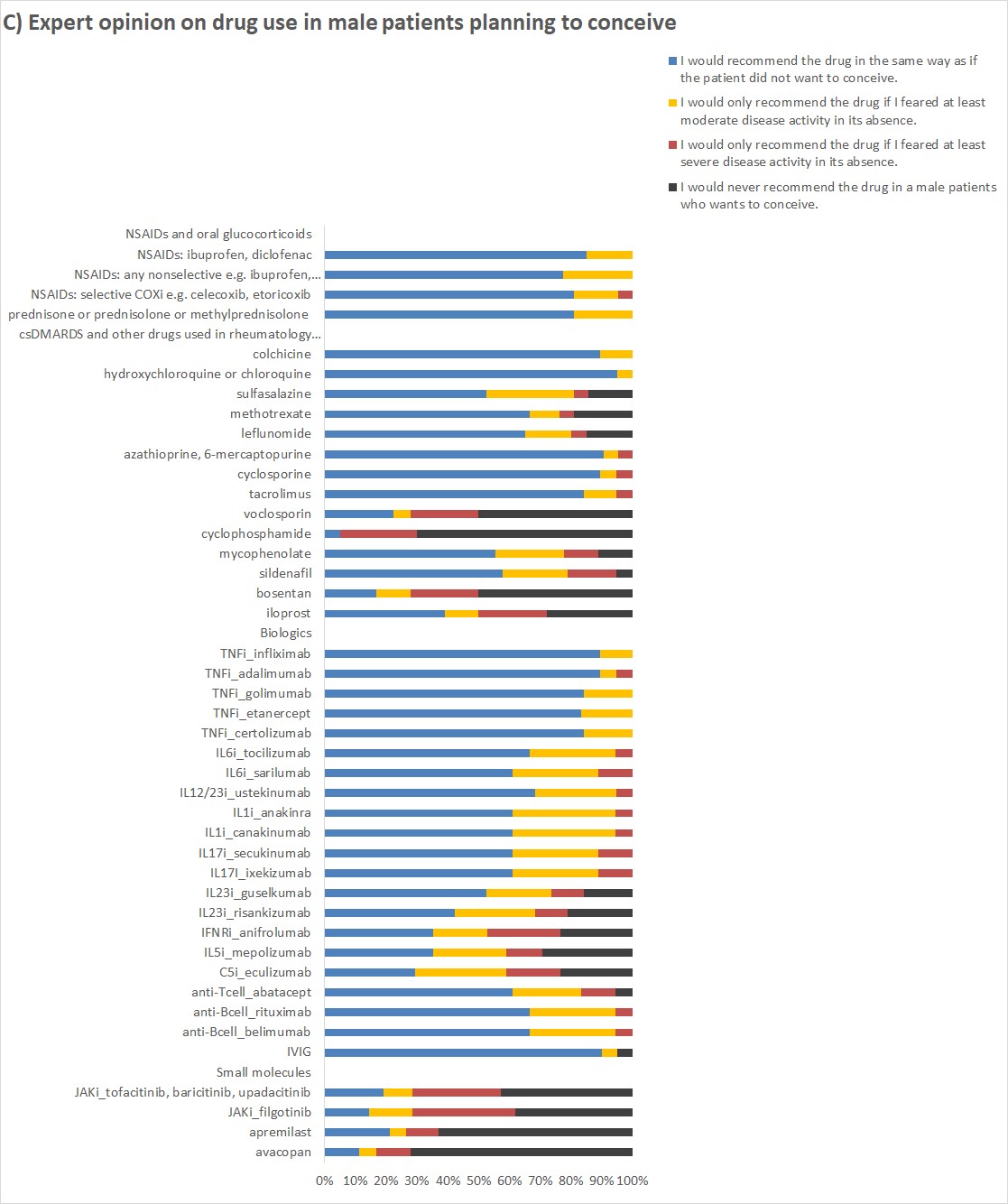
In male patients planning to conceive most antirheumatic drugs were recommended in the same way as outside family planning (Figure 1C).
Conclusion: Experts in the field base their recommendations for the use of antirheumatic drugs in women before and during pregnancy primarily on the evidence of drug safety and the potential risk of active disease for the mother and the fetus. The expert’s risk benefit analysis is most obvious for the recommendations on the use of biologics in women planning a family. On the other hand, a more permissive recommendation of antirheumatic drugs is possible for breastfeeding women and men planning to conceive due to the absence of fetal exposure.
Among the current EULAR task force, 21 experts were drug prescribers and replied to a survey on drug use in women before and during pregnancy, breastfeeding women and male patients planning to conceive.
A) Answers to the question about drug use before or during pregnancy: What would you recommend to a woman under this medication who wants to conceive or already has a positive pregnancy test?
To cite this abstract in AMA style:
Pluma A, Rüegg L, Hamroun S, Finckh A, Meissner Y, Foerger F. Risk of Active Disease Determines the Expert Opinion Use of Biologics in Pregnancy, but Less in Breastfeeding, and in Men Planning a Family [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/risk-of-active-disease-determines-the-expert-opinion-use-of-biologics-in-pregnancy-but-less-in-breastfeeding-and-in-men-planning-a-family/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-active-disease-determines-the-expert-opinion-use-of-biologics-in-pregnancy-but-less-in-breastfeeding-and-in-men-planning-a-family/