Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Risk factors for treatment failure in patients with giant cell arteritis (GCA) are poorly understood. The objective of this analysis was to identify predictors of treatment failure in GCA patients receiving tocilizumab (TCZ) or placebo (PBO) in combination with prednisone in a randomized controlled trial (GiACTA).1
Methods: Two hundred fifty GCA patients received weekly or every-other-week TCZ plus a 26-week prednisone taper (TCZ+pred) or PBO plus a 26- or 52-week prednisone taper (PBO+pred). Patients who achieved and maintained clinical remission (CR) from week 12 to week 52 while adhering to the protocol prednisone taper were classified as responders. CR, adjudicated by investigators, was defined as the absence of disease flare (GCA signs or symptoms, and/or ESR elevation attributable to GCA that required further treatment [eg, rescue prednisone]) regardless of C-reactive protein (CRP) level. Treatment failure was defined as failure to achieve CR by week 12 or occurrence of flare between weeks 12 and 52. Both TCZ groups and both PBO groups were combined for this analysis. Potential predictors investigated included baseline demographics, disease- and treatment-related factors, and health-related quality of life (HRQOL) measures. Univariate and multivariate analyses were performed.
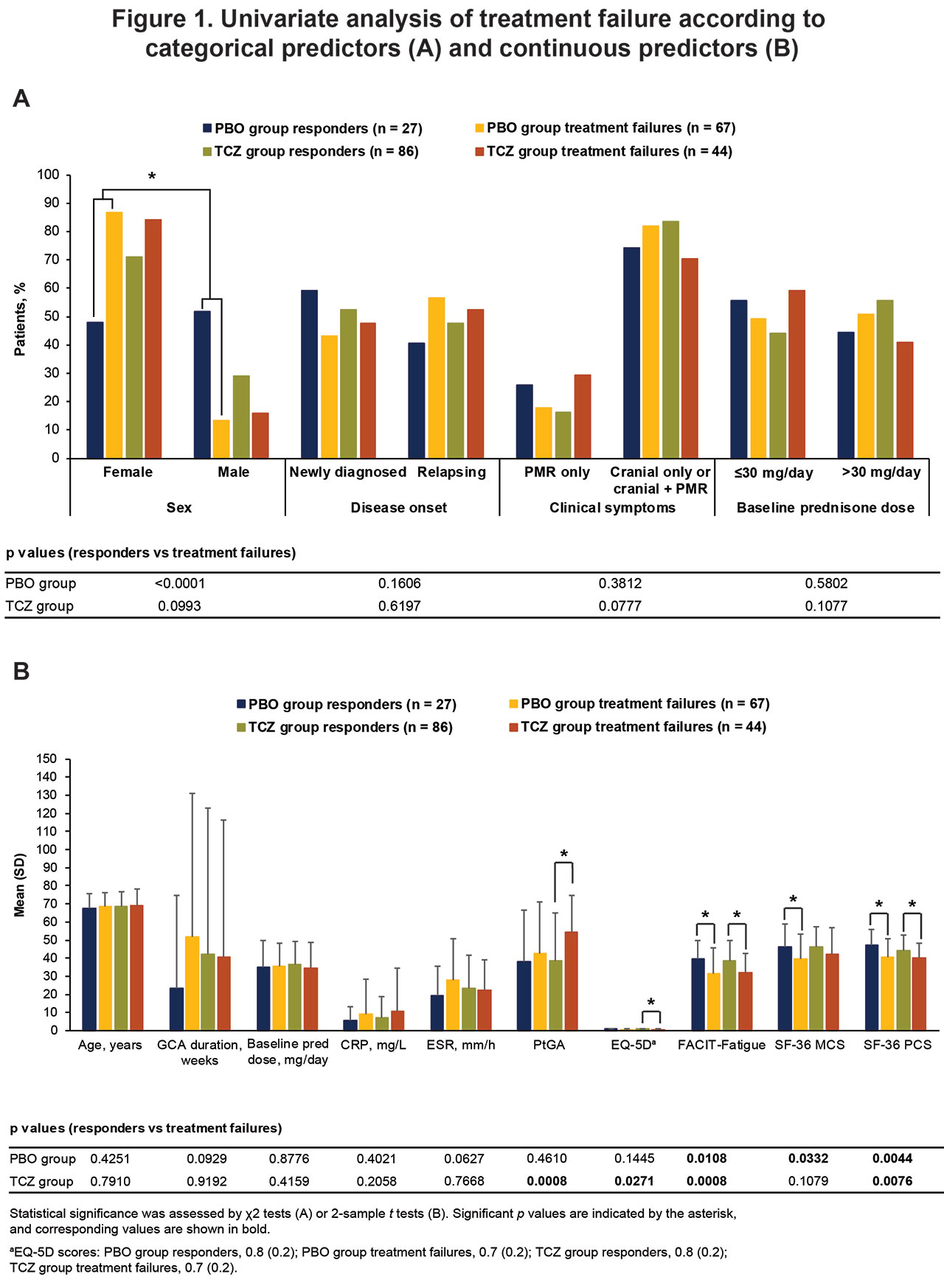
Results: Overall, 45% (113/250) of patients were responders: 27% (27/101) in the PBO+pred groups and 58% (86/149) in the TCZ+pred groups. In contrast, 44% (111/250) of patients experienced treatment failure: 66% (67/101) in the PBO+pred group and 30% (44/149) in the TCZ+pred group. The other 10% (26/250) of patients were nonresponders for reasons other than treatment failure: 7 in the PBO+pred group and 19 in the TCZ+pred group. In univariate analysis, female sex and lower baseline SF-36 Physical Component Summary (PCS), Mental Component Summary, and FACIT-Fatigue scores were associated with treatment failure among PBO+pred–treated patients, whereas higher patient global assessment of disease activity scores and lower SF-36 PCS, FACIT-Fatigue, and EQ-5D scores were associated with treatment failure among TCZ+pred–treated patients (Figure 1). Among TCZ+pred–treated patients, no treatment response difference according to sex was observed. Age, previous relapse, starting prednisone dose, and GCA clinical features (cranial or polymyalgia rheumatica symptoms) were not associated with treatment failure in either group based on univariate analysis. Multivariate logistic regression demonstrated that PBO+pred treatment, female sex, and worse FACIT-Fatigue scores at baseline increased the risk for treatment failure (Figure 2).
Conclusion: Female GCA patients responded particularly poorly if treated with prednisone alone according to univariate analysis. Female sex, impaired HRQOL at baseline, and treatment with prednisone alone are risk factors for treatment failure in GCA. These factors may be considered when determining which treatment would be best for a particular patient.
Reference: 1. Stone JH et al. N Engl J Med 2017;377:317-328.
To cite this abstract in AMA style:
Unizony S, Bao M, Han J, Luder Y, Sidiropoulos P, Pei J, Stone J. Risk Factors for Treatment Failure in Patients with Giant Cell Arteritis Treated with Tocilizumab Plus Prednisone versus Prednisone Alone [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-factors-for-treatment-failure-in-patients-with-giant-cell-arteritis-treated-with-tocilizumab-plus-prednisone-versus-prednisone-alone/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-treatment-failure-in-patients-with-giant-cell-arteritis-treated-with-tocilizumab-plus-prednisone-versus-prednisone-alone/