Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatologic disorders can take years to diagnose. Diagnosis often requires a combination of specific symptoms, examination findings and laboratory testing, rather than a single test. Additionally, symptoms presenting in rheumatologic processes are often present in other common conditions, making diagnosis challenging. A common starting point is an antinuclear antibody (ANA) test, often ordered by a non-rheumatology provider, followed by a wide range of further antibody testing to narrow disorders. Here, we sought to examine patterns of post-ANA testing using real world evidence to inform order guidance opportunities and help advance diagnosis.
Methods: De-identified data from the Labcorp® testing databasewere queried for all ANA orders (both individual and panel testing) between 2011 and 2022. Any ANA order placed by a rheumatologist was excluded. Follow-up antibody testing (such as Anti-Smith, Anti-dsDNA antibodies, etc.) within 365 days post-positive ANA result (1:80 or higher) and respective positivity rates were examined. Reason for ANA ordering was evaluated via ICD-10 codes associated with the original order where the ANA was the only test run on an individual specimen to remove any conflicting ICD codes with other tests.
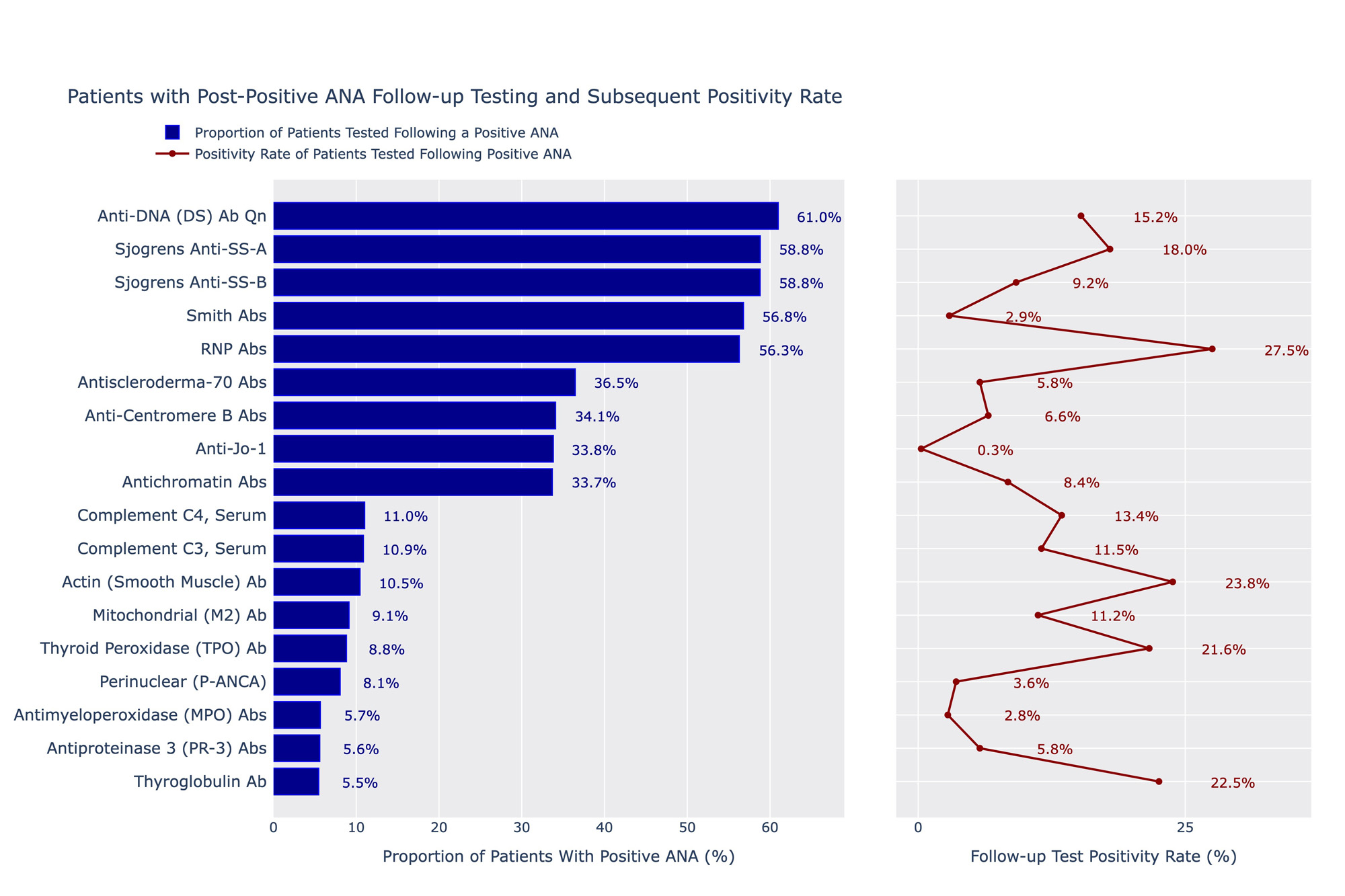
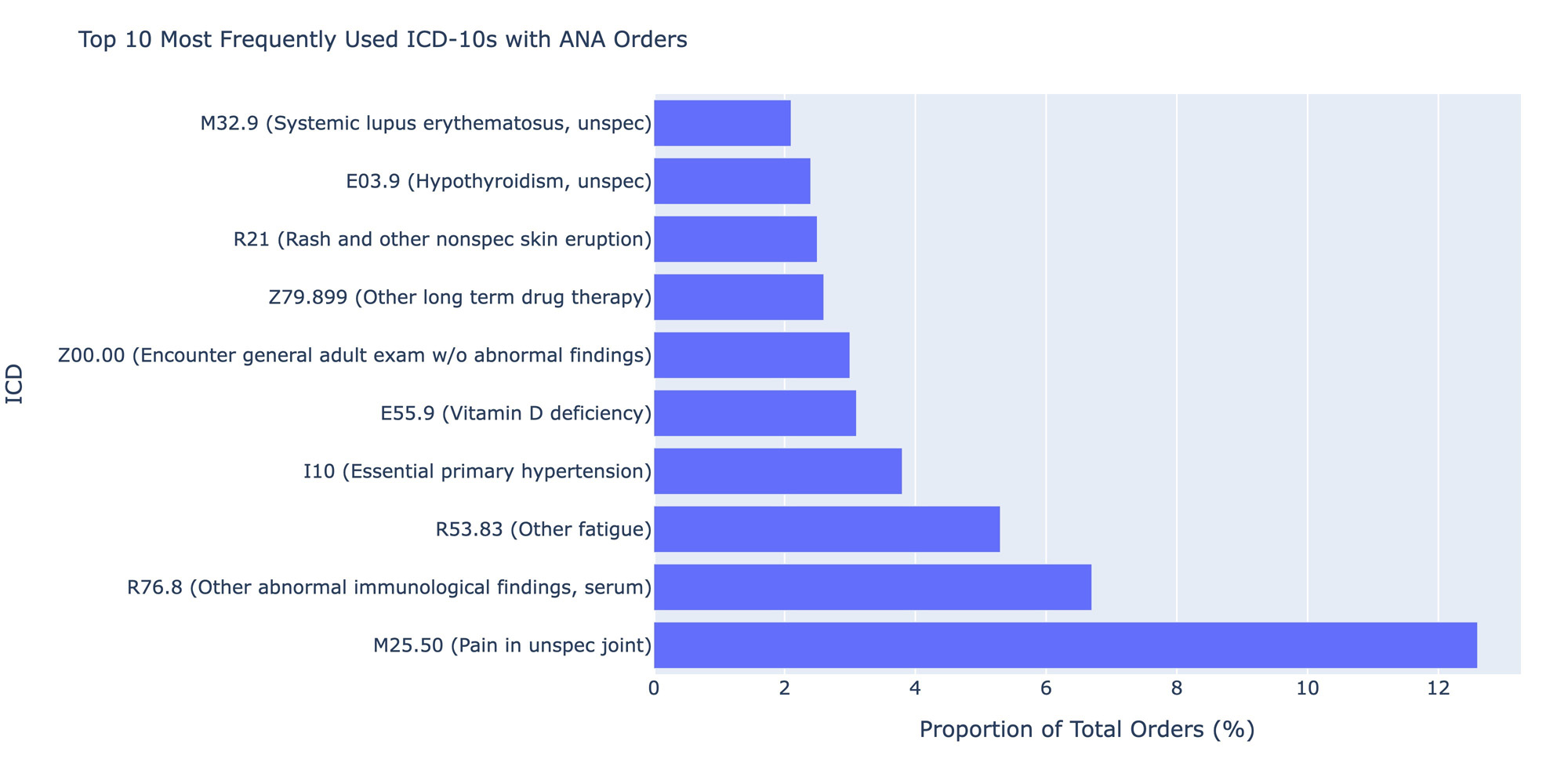
Results: A total of 1,834,048 distinct ANA tests were ordered by a non-rheumatology provider between 2011 and 2022 and had positive results. Of these tests, 49.7% (n=910,683) were ordered by primary care and only 11.2% of the total sample (n=204,608) were associated with patients who had any order placed by a rheumatologist within a year of the ANA. Anti-dsDNA, anti-SSA, anti-SSB, Smith and RNP antibodies were the most frequently ordered autoimmune-specific follow-up tests performed by non-rheumatologists, each representing just over 50% of the sample. Of these, RNP antibodies had the highest positivity rate (27.5%), and Smith antibodies the lowest (2.9%). Thyroglobulin antibodies were the least ordered tests (5.5% of cohort) but had one of the highest positivity rates (22.5%). Of specimens with only an ANA ordered (n=148,882), M25.50 (pain in unspecified joint, 12.6% of the cohort), R76.8 (other abnormal findings in serum, 6.7%) and R53.83 (other fatigue, 5.3%), I10 (essential primary hypertension, 3.8%) and E55.9 (Vitamin D deficiency, 3.1%) were the most frequent ICD-10s ordered but together represented only 8.1% of the total cohort.
Conclusion: There does not appear to be a clear ordering pattern of follow-up testing within a year of a positive ANA test. While more than half of the cohort received some common antibody tests like Anti-dsDNA, positivity rates were low. Interestingly, approximately 10% of the cohort had an order from a rheumatologist within a year, suggesting the majority of diagnostic workup is being done by primary care. Ordering ICD-10 codes represent suspicion for a rheumatologic process and symptoms, such as joint pain, but the frequency of hypertension and Vitamin D deficiency codes do not readily explain a reason for ordering. Results suggest the need for a guideline approach for rheumatologic disorder testing evaluation and education in the non-rheumatologic setting.
To cite this abstract in AMA style:
Alfego D, Hlatky Q, Naides S, Lee K, Ennis J, Clark K. Rheumatologic Disorder Diagnostic Testing Patterns:Real World Evidence from a National Laboratory Database [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/rheumatologic-disorder-diagnostic-testing-patternsreal-world-evidence-from-a-national-laboratory-database/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatologic-disorder-diagnostic-testing-patternsreal-world-evidence-from-a-national-laboratory-database/