Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Monoclonal antibodies targeting co-inhibitory immune checkpoints (PD-1/PDL1 axis or CTLA-4) showed unprecedented clinical activity in several types of cancer by restoring antitumor immune responses. We recently described that vasculitis (giant cell arteritis) was a rheumatic immune-related adverse event (irAE) but the description of other rheumatic adverse events remains sparse.
Methods: Using WHO’s global database of individual case safety reports, we compared rheumatic adverse events reporting in immune checkpoint inhibitors (ICI) exposed patients with the reporting in the full database. All rheumatic irAEs (except vasculitis) were included (according to the Medical Dictionary for Regulatory Activities). The association between ICI and rheumatic adverse events was analyzed using the reporting odds ratio (ROR) and the information component (IC: an indicator value for disproportionate reporting observed vs expected values). A positive IC025 (the lower end of the IC 95% credibility interval) is considered to be significant.
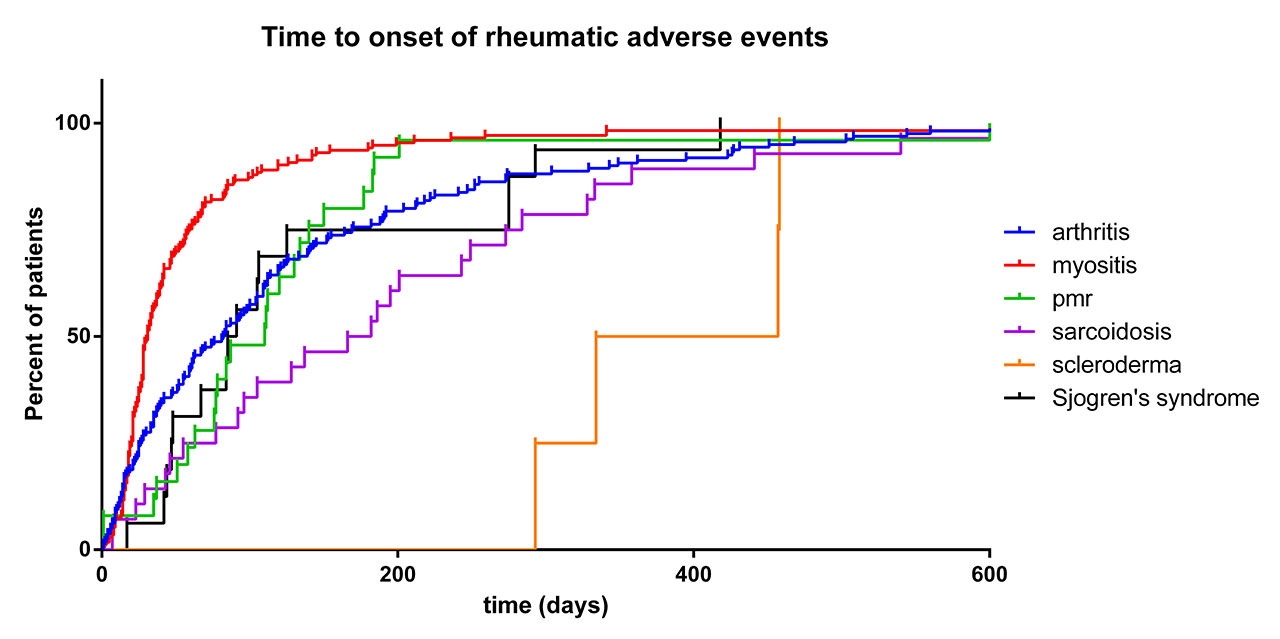
Results: We identified 54,416 adverse events reported in patients treated with ICI (14,988,450 adverse events reported in patients treated with any drugs, full database since 2008). Compared to the full database, ICI treatment was associated with higher reporting of arthritis (121,811 reports for the full database vs 606 for ICI, ROR 1.4 [1.3-1.5]; IC025 0.34), myositis (26,722 vs 465, ROR 4.9 [4.5-5.4]; IC025 2.12), sarcoidosis (2,772 vs 94, ROR 9.6 [7.9-11.9]; IC025 2.85), polymyalgia rheumatica (1,504 vs 76, ROR 14.6 [11.6-18.4]; IC025 3.34), Sjogren’s syndrome (2,000 vs 49, ROR 6.9 [5.2-9.2]; IC025 2.24) and scleroderma (2,385 vs 17, ROR 2.0 [1.2-3.2]; IC025 0.17). Myositis patients were more frequently male (70.4%; p< 0.0001 vs 56% in other rheumatological irAE patients) and treated with anti-PD1/L1 (ROR 2.8 [1.9-4.0] vs anti-CTLA4) or with ICIs combination (ROR 2.0 [1.6-2.6] vs monotherapy). Myositis occurred earlier compared to other rheumatic irAEs (time to onset: 31 IQR [19.5-58.5] days) and were associated with the highest mortality rate (30.3%, p< 0.0001). Arthritis and Sjogren’s syndrome had similar time to onset (78.5 [25-166.5] and 88 [47.8-145.8] days, respectively), and were associated with a mortality of 6.9 and 0%. Arthritis and Sjogren’s syndrome occurred more frequently in patients with anti-PD1/L1 (vs anti-CTLA4; ROR 2.1 [1.6-2.8] or 4.4 [1.0-18.1]) with ICI combination (vs monotherapy; ROR 1.6 [1.3-2.0] and 2.9 [1.5-5.6]) whereas polymyalgia rheumatica was more frequently reported in patients with anti-PD1/L1 monotherapy (ROR 5.6 [1.8-17.7]). Sarcoidosis occurred in younger patients (57 [48-65.3] years) and with a longer time to onset (174 [71.5-275.8] days; p< 0.001). Scleroderma had the longest time to onset (395.5 [323.8-457.2] days; p< 0.001).
Conclusion: Among rheumatic disorders in addition to vasculitis: arthritis, myositis, sarcoidosis, polymyalgia rheumatica, Sjogren’s syndrome and scleroderma have a higher reporting. Patients characteristics, type of ICI, time to onset, and patient outcome depend on the type of rheumatic irAEs. Myositis is the most severe.
To cite this abstract in AMA style:
Anquetil C, Benveniste O, Moslehi J, Johnson D, lebrun-vignes B, Lambotte O, Selam J, Spano J, Allenbach Y. Rheumatic Toxicities Associated with Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/rheumatic-toxicities-associated-with-immune-checkpoint-inhibitors-an-observational-retrospective-pharmacovigilance-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatic-toxicities-associated-with-immune-checkpoint-inhibitors-an-observational-retrospective-pharmacovigilance-study/