Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICIs) have led to improved outcomes in multiple cancers, but they are associated with immune-related adverse events (irAEs), including inflammatory arthritis. We report our single center experience with rheumatic irAEs.
Methods: Patients ≥18 years treated with an ICI at our institution from 2011-2018 and seen by rheumatology for evaluation of musculoskeletal symptoms following ICI were included. A rheumatologist evaluated all patients and confirmed the diagnosis of irAE at the time of evaluation. Cases were reviewed by two rheumatologists to confirm the presence of a rheumatic irAE. Inflammatory arthritis was defined as joint pain with inflammatory features on history and physical exam without an alternative etiology. PMR was based on 2012 ACR/EULAR criteria. Patients with and without pre-existing rheumatic conditions were analyzed.
Results: Twenty-nine patients were evaluated by rheumatology for suspected rheumatic irAEs. Eighteen had confirmed inflammatory arthritis (n=12) or PMR (n=6) (Table 1). Six had inflammatory joint pain but no evidence of synovitis by exam, imaging or synovial fluid. Five had an alternative etiology identified (gout, fracture, infectious arthritis).
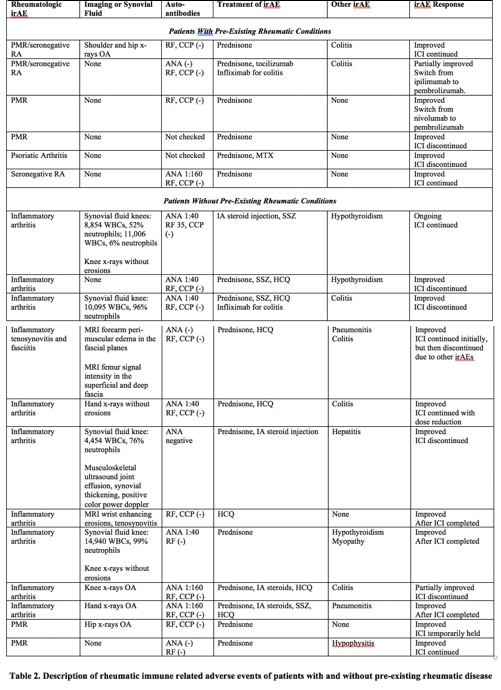
Patients with pre-existing rheumatic conditions (n=6) (Table 2) developed a flare of their underlying disease a median of 12 weeks following ICI (range 1-43 weeks). The mean initial prednisone dose was 25mg/day (range 15-40mg), with a mean duration of use of 37.4 weeks (range 17-57 weeks). One patient with PMR/RA required tocilizumab; one patient with PsA required MTX. Two patients discontinued ICI.
Ten patients without a pre-existing rheumatic condition developed inflammatory arthritis and two developed PMR a median of 36 weeks following ICI (range 9-160 weeks, p=0.0485 compared to patients with pre-existing conditions). Patients with PMR were treated with prednisone. Patients with inflammatory arthritis were treated with systemic or IA steroids and in 8 cases with DMARDs, including HCQ or SSZ. The mean initial prednisone dose was 31.1mg/day (range 6-60mg), with a mean duration of use of 26.6 weeks (range 3-82 weeks). ICIs were discontinued in 5 patients.
Conclusion: We report 18 patients with inflammatory arthritis or PMR after ICI treatment. Our work highlights the differences between patients with and without pre-existing rheumatic conditions and describes the successful use of non-biologic DMARDs as treatment. Further studies are needed to determine the incidence of rheumatic irAEs, to improve classification and to identify the most effective treatment.
To cite this abstract in AMA style:
Nasrallah M, Mooradian M, Miloslavsky E, Cohen J, Gainor J, Lawrence D, Reynolds K, Kohler M, Sullivan R, Schoenfeld S. Rheumatic Complications of Immune Checkpoint Inhibitor Therapy: A Single Center Experience [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/rheumatic-complications-of-immune-checkpoint-inhibitor-therapy-a-single-center-experience/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatic-complications-of-immune-checkpoint-inhibitor-therapy-a-single-center-experience/