Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Belimumab is approved as add-on therapy in active, autoantibody-positive SLE. Safety and efficacy up to 13 years has been reported in adults, but potential for rebound upon temporary discontinuation of belimumab has not been investigated. This study (BEL116027; NCT02119156) assessed the impact of temporary withdrawal of intravenous (IV) belimumab.
Methods: In this 52-week open-label study, adults with SLE who received belimumab 10 mg/kg IV for ≥6 months in open-label studies were recruited to three arms: treatment holiday (TH; 24-week belimumab withdrawal, reintroduction for 28 weeks); continuous belimumab (treatment control; TC), and long-term discontinuation (LTD) (Figure).
The primary endpoint was time to first SLE flare (SFI). Secondary endpoints included flare rates and evidence of rebound (SELENA-SLEDAI [SS] exceeding parent study baseline score, anytime post baseline to Week 24). Anti-drug antibodies (ADAs) and changes in immunoglobulins and B Cells were measured. Safety was monitored.
Due to slow enrollment the study was amended to reduce sample size; all analyses are descriptive.
Results: The ITT comprised 80 patients (TH: n=12, TC: n=29; LTD: n=39). The majority were female (88.8%), ≤45 years of age (71.3%), and had SS >3 at baseline in the parent study (97.5%). Day 0 disease activity, race and geographic distribution differed for LTD vs TC/TH. A higher proportion of LTD patients (8/39; 20.5%) had flares during screening for this study vs TH (1/12; 8.3%) or TC arms (0/29; 0%). Also, 41.0% of LTD patients were recruited from the US and 23.1% were White; TC and TH arms were recruited from China, Korea and Japan (100% Asian).
The majority in the holiday phase did not flare and flare rate during this time (1.0) was comparable to TC (0.6) (Table 1). Flare rate was highest in LTD (2.1). There were 9 severe and 4 renal flares in LTD and none in TH/TC.
No TH patients rebounded; two TC and two LTD patients rebounded (Table 1).
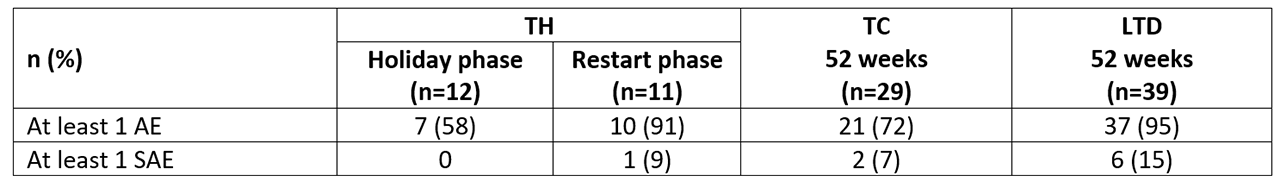
Adverse event (AE) and serious AE (SAE) rates were higher in LTD vs TC (Table 2). There were no withdrawals due to AEs and no SAEs in the holiday phase.
No ADAs were detected. In all arms, median levels of anti-dsDNA remained suppressed and complement C3/C4 levels remained above baseline over 52 weeks. At Day 0, median immunoglobulin levels were below baseline relative to the parent study and remained decreased throughout for all arms. B Cell levels remained depleted in TC throughout; in TH and LTD, CD19, CD20, and naïve B Cell counts started to recover after 16 weeks off-treatment but rapidly decreased to minimal levels following restart (TH). No Grade 3/4 laboratory abnormalities were noted in the TH arm. A small proportion of patients in the TC and LTD arms had Grade 3/4 laboratory abnormalities.
Conclusion: Temporary (24-week) discontinuation of belimumab in patients with low disease activity did not appear to increase risk of SLE flares or rebound. Across 52 weeks, no ADAs were identified in any arm and the belimumab safety profile was consistent with previous reports. Small sample size and differences in Day 0 characteristics between LTD and TC arms limit the ability to draw inferences.
Study funding: GSK. Medical writing support: C Brazaitis, PhD, Fishawack Indicia Ltd, UK (funded by GSK).
To cite this abstract in AMA style:
Bae S, Dimelow R, Ji B, Kurrasch R, Muzaffar S, Punwaney R, Roth D, Stober P, Song Y, Xie W, Zhang F. Results of the Open-label, Non-randomized 52-Week Study to Evaluate Treatment Holidays and Rebound Phenomenon After Treatment with Belimumab in Patients with SLE [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/results-of-the-open-label-non-randomized-52-week-study-to-evaluate-treatment-holidays-and-rebound-phenomenon-after-treatment-with-belimumab-in-patients-with-sle/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/results-of-the-open-label-non-randomized-52-week-study-to-evaluate-treatment-holidays-and-rebound-phenomenon-after-treatment-with-belimumab-in-patients-with-sle/