Session Information
Date: Sunday, November 8, 2015
Title: Osteoporosis and Metabolic Bone Disease - Clinical Aspects and Pathogenesis Poster
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Romosozumab is a sclerostin inhibitor that rapidly increases bone
mineral density (BMD) through a dual effect on bone, causing increased bone
formation and decreased bone resorption, as shown in a global phase 2 study in postmenopausal
women with low bone mass (McClung et al NEJM 2014). Here we report the
key results of a phase 2 dose-ranging study to assess the efficacy and safety
of romosozumab in a Japanese population (NCT01992159).
Methods:
This randomized,
double-blind, placebo-controlled study enrolled Japanese postmenopausal women
aged 55–85 years with a lumbar spine (LS), total hip (TH), or femoral neck (FN)
DXA T‑score ≤ –2.5. Women were randomized to receive placebo or
1 of 3 doses of subcutaneous romosozumab (70 mg, 140 mg, or 210 mg) once monthly
(QM) for 12 months. The primary endpoint was percentage change from baseline in
LS BMD at month 12. Secondary endpoints included percentage change from
baseline in LS BMD at month 6, TH and FN BMD at months 6 and 12, and percentage
change from baseline in serum bone turnover markers.
Results:
Women enrolled in
the study (N = 252) had a mean (standard deviation) age of 68 (6.4) years and
mean LS, TH, and FN T-scores of –2.7, –1.9, and –2.3, respectively. All
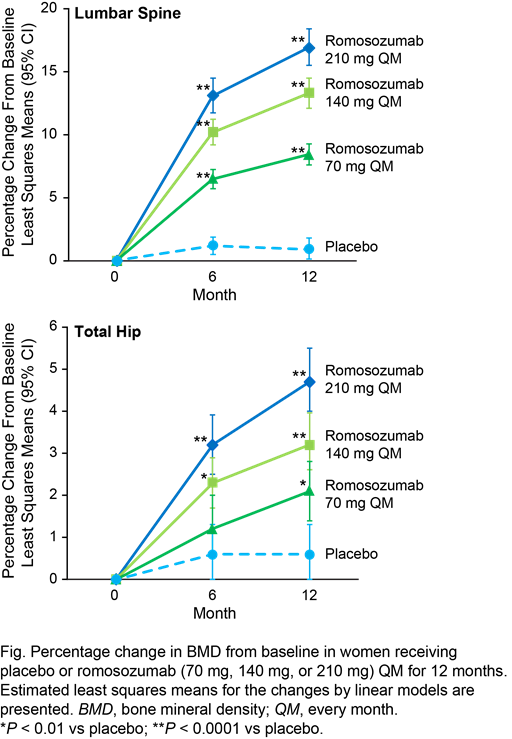
romosozumab doses significantly increased BMD compared with placebo at each of
the three skeletal sites at month 12 (P < 0.01). The largest
improvements were observed with romosozumab 210 mg QM, which resulted in BMD
gains from baseline of 16.9% and 4.7% at the LS and TH, respectively (Fig.),
and 3.8% at the FN, at month 12. All
doses of romosozumab increased P1NP and reduced CTX vs placebo by week 1 (P < 0.0001). In
the 210 mg QM group, P1NP levels peaked at month 1 (median increase 101.1%) and
fell below placebo levels by month 12; CTX levels were
lowest at week 1 (median decline 45.6%) and were still below placebo at month 12. Subject incidences of adverse
events and serious adverse events were generally comparable between treatment
groups. Numerical differences were observed between the total romosozumab vs placebo
groups for subject incidence of mild to moderate osteoarthritis adverse events (6.9%
vs 0%) and mild injection-site reactions (3.7% vs 1.6%).
Conclusion:
In this study of
Japanese women with postmenopausal osteoporosis, romosozumab treatment resulted
in rapid, large, and significant gains in BMD compared with placebo and
baseline, and the 210 mg QM dose showed the greatest efficacy. Romosozumab was
generally well tolerated in this population. Global phase 3 studies evaluating
the 210 mg QM dose for the treatment of osteoporosis are underway.
To cite this abstract in AMA style:
Ishibashi H, Crittenden D, Miyauchi A, Libanati C, Maddox J, Chen L, Grauer A. Results of a Phase 2 Clinical Trial to Evaluate the Effects of Romosozumab in Japanese Women with Postmenopausal Osteoporosis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/results-of-a-phase-2-clinical-trial-to-evaluate-the-effects-of-romosozumab-in-japanese-women-with-postmenopausal-osteoporosis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/results-of-a-phase-2-clinical-trial-to-evaluate-the-effects-of-romosozumab-in-japanese-women-with-postmenopausal-osteoporosis/