Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: OMERACT (OM) recently developed preliminary flare questions (PFQs) that capture domains important to patients (pts) to identify a flare of RA1. Whether these change in pts who flare is unknown.
Objectives: To study responsiveness to change of the PFQs’ 9 domains within early RA (ERA) pts who have a flare.
Methods: Paired data from CATCH (Canadian early arthritis cohort) pts who prospectively answered the PFQs twice in 3 months between 12/2011-5/2013 were used. The PFQs comprise 7 pt reported outcomes (PROs) assessing pt global, pain, physical function, stiffness, fatigue, coping, and participation as questions scored from 0-10 (best-worst) and pt reported tender and swollen joint counts (PtTJC40, PtSJC40). The median change (IQR) and effect size (ES) for each domain was compared in pts who flared based on a worsening of DAS28 of 1.2 (or 0.6 if DAS28> 3.2)2 (DASdef) or by answering “Are you having a flare at this time?” with a flare defined as changing from no to yes (Fdef). Using the last pairs of 3-month interval PFQs, changes in PROs were analyzed by the Wilcoxon signed rank test. Responsiveness to change in each of the PFQs for flare vs. no-flare was assessed by non-parametric effect sizes (ES) calculated for both definitions.
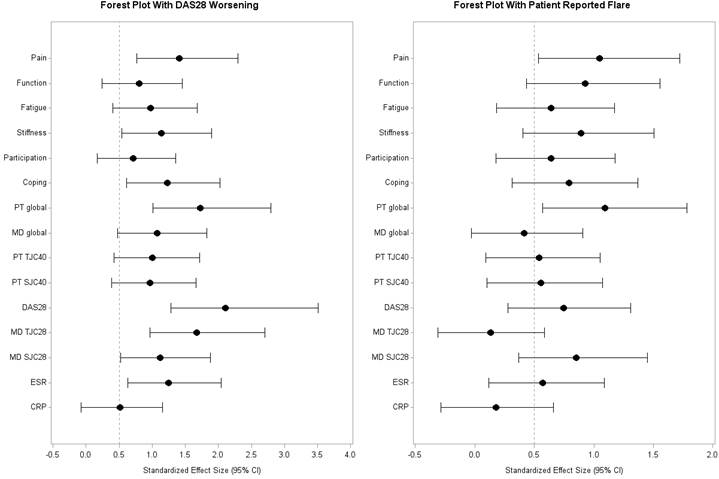
Results: Of 115 eligible pts 79% were female, 85% Caucasian, 90% met ACR/EULAR 2010 criteria, 17% smoked, 24% had erosions, 69/73% were ACPA/RF+ve. Pts had a mean (SD) age of 55 (15) years, symptom duration 5.4 (3.0) months; Pts’ initial DAS28 was 3.03 (1.34), HAQ-DI 0.52 (0.55); 46%/15% were in DAS28 remission/low disease activity. 23% reported a flare with a mean (SD) intensity of 6.3 (2.4) and duration ≥ 8 days in 59%. Median (IQR) increases in DAS28 using DASdef and Fdef was 1.8(1.-1.2) and 0.6(.4-.35). Changes in PtGlobal, pain, function, fatigue, stiffness, coping, PtTJC40 and PtSJC40 all changed significantly for flare vs. non-flare pts (Table). Responsiveness to change differed according to flare definitions (Figure).
Conclusion: The OM PFQs are responsive to change with worsening of disease activity within ERA pts over time but the magnitude of change varies with flare definition. Studies are needed to understand the relative contributions of each domain to identify RA flares that necessitate treatment increases.
Figure: Effect Size (adjusted 95% CI) for each domain in the PFQs according to two definitions of RA flare.
Table: Responsiveness of OMERACT PFQ Domains and Physician/Lab based variables reported as median change (IQR) and effect size (p-value) in flare and non-flare groups using 2 flare definitions.
|
Variables |
DAS definition (increase of 1.2 or >.6 if 3.2) |
Patient Flare definition (No to Yes) |
||||
|
Flare* |
Non-flare* |
Effect Size** (p-value)*** |
Flare* |
Non-flare* |
Effect Size** (p-value)*** |
|
|
N = 16 |
N = 99 |
N = 26 |
N = 89 |
|||
|
Pain |
2 (2.5) |
0 (2) |
1.41 (<0.0001) |
2 (2) |
0 (2) |
1.05 (<0.0001) |
|
Function |
1.5 (3.5) |
0 (2) |
0.8 (0.0043) |
1.5 (3) |
0 (1) |
0.93 (0.0001) |
|
Fatigue |
1.5 (3.0) |
0 (2) |
0.97 (0.0006) |
1 (3) |
0 (2) |
0.64 (0.0056) |
|
Stiffness |
3.0 (4.5) |
0 (2) |
1.14 (<0.0001) |
1.5 (4) |
0 (1) |
0.89 (0.0002) |
|
Participation |
1.5 (4.5) |
0 (1) |
0.71 (0.0103) |
0 (3) |
0 (1) |
0.64 (0.0057) |
|
Coping |
3.0 (3.0) |
0 (1) |
1.22 (<0.0001 |
1 (3) |
0 (1) |
0.79 (0.0008) |
|
Pt global |
3.0 (3.0) |
0 (2) |
1.72 (<0.0001) |
1.5 (3) |
0 (1) |
1.09 (<0.0001) |
|
PT TJC40 |
4.0(12.0) |
0 (5) |
1 (0.0004) |
1 (6) |
0 (5) |
0.54 (0.0179) |
|
PT SJC40 |
2.0 (7.0) |
0 (2) |
0.96 (0.0007) |
0 (4) |
0 (2) |
0.55 (0.0154) |
|
|
||||||
|
Physician global |
0.1 (2) |
0 (1.5) |
1.08 (0.0003) |
0 (2) |
0 (1.4) |
0.41 (0.0677) |
|
DAS28 |
1.5 (1) |
-0.2(1.3) |
2.11 (<0.0001) |
0.6 (1.6) |
-0.2 (1.3) |
0.75 (0.0014) |
|
CRP |
0.2 (3) |
0 (2.9) |
0.51 (0.0881) |
0 (3.7) |
0 (2.9) |
0.18 (0.4544) |
|
MD TJC28 |
3 (5.5) |
0 (2) |
1.67 (<0.0001) |
0 (4) |
0 (2) |
0.13 (0.5563) |
|
MD SJC28 |
0.5 (2) |
0 (1) |
1.12 (0.0001) |
0 (1) |
0 (1) |
0.85 (0.0003) |
|
ESR |
6.5 (6.6) |
-2 (7) |
1.24 (<0.0001) |
4 (8) |
-2 (7) |
0.57 (0.0129) |
|
Responsiveness of PFQ Domains & Physician/Lab based variables (*Median change (IQR), **Effect Size (ES) (non-parametric), ***p-value using Wilcoxin Rank Sum Test. This analysis did not incorporate the self-management domain. |
||||||
Disclosure:
V. P. Bykerk,
None;
K. Visser,
None;
E. Choy,
None;
C. O. Bingham III,
None;
D. Lin,
None;
J. Xiong,
None;
G. Boire,
None;
B. Haraoui,
None;
C. A. Hitchon,
None;
J. E. Pope,
None;
J. C. Thorne,
None;
D. Tin,
None;
E. C. Keystone,
None;
S. J. Bartlett,
None;
CATCH Investigators,
Amgen Canada Inc., Pfizer Canada Inc.,
2,
Hoffmann-LaRoche Ltd., UCB Canada Inc., Bristol-Myers Squibb Canada Co., AbbVie Corporation (formerly Abbott Laboratories Ltd.), and Janssen Biotech Inc. (a wholly owned subsidiary of Johnson & Johnson Inc.),
2;
OMERACT Flare Working Group,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/responsiveness-of-the-outcomes-measures-in-rheumatology-clinical-trials-initiatives-preliminary-flare-questions-to-detect-flares-within-patients-in-the-canadian-early-arthritis-cohort/