Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Most randomized controlled trials (RCTs) with biologic medicines in systemic lupus erythematosus (SLE) failed to reach their respective end-points with the rates of the response to placebo (plus standard of care treatment) being unexpectedly high. The aim of this systematic review was to quantify the response to placebo for different end-points in the RCTs in non-renal, non-neuropsychiatric lupus patients.
Methods: The Pubmed database was searched from 2000 to April 2019 for phase II/III RCTs that assessed the efficacy and safety of biologic drugs in human non-renal, non-neuropsychiatric SLE at 48 or 52 weeks after randomization. Data on efficacy (primary and secondary end-points) and safety (serious adverse events, serious infections, malignancies and deaths) of the placebo-treated patients were collected in a pre-established data retrieval form. Descriptive statistics were used.
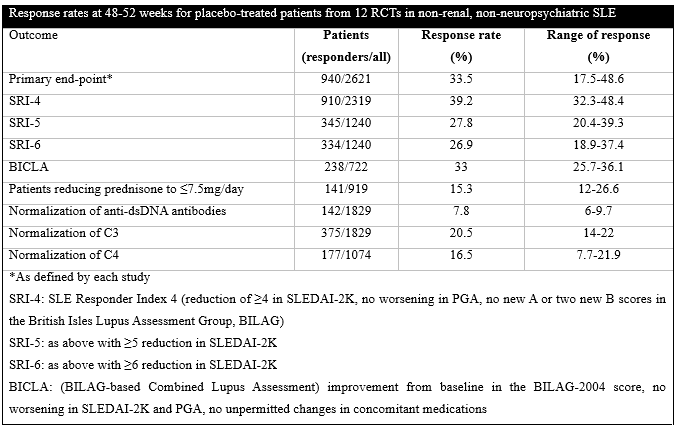
Results: Twelve RCTs (n=7940 in total) were included. Patients who received placebo (n=2621) were mostly females (2460, 93.9%) and Caucasians (1621, 61.8%) with a mean age of 39.7±12.2 years and mean disease duration of 6.5±6.8 years. Their initial SLE Disease Activity Index 2000 (SLEDAI-2K) was 10.3±3.7 whereas 1278/2096 (61%) were positive for anti-dsDNA antibodies, 881/2039 (43.2%) had low C3 and 772/2039 (37.9%) low C4 at randomization. Their standard of care (SOC) treatment included glucocorticosteroids in 2128/2464 (86.4%) [mean dose 11.5±7.9mg/day, prednisone >7.5mg/day in 1062/1917, 55.4%], antimalarials in 1619/2376 (68.1%) and immunosuppressives in 1067/2376 (44.9%) (509 azathioprine, 369 methotrexate, 270 mycophenolate). There were no significant differences regarding demographics, clinical manifestations and treatment between placebo-treated patients and individuals in the active arms of most RCTs. The response rates of the placebo-treated patients for the different end-points are shown in the table.
The mean reduction in SLEDAI-2K (n=1267) was 4.05 (range 3.3-4.8) and in the Physician Global Assessment (PGA, 0-100 scale, n=1554) 19.9 (range 11.7-23.8). The sample size in 10 studies was calculated with an expectation of an average 15% difference between the active drug and placebo (range 12-20%) in achieving the primary end-point; the actual difference was 9% on average (range 1.2-17.9%). Regarding safety, there were 449/2621 (17.1%) serious adverse events with 105/1899 (5.5%) serious infections, 6/1310 (0.5%) malignancies and 17/2621 (0.65%) deaths.
Conclusion: One third of the patients treated with placebo plus SOC achieved their respective primary end-points in RCTs with biologics in non-renal, non-neuropsychiatric SLE. The response rate was even higher for certain end-points, such as the SRI-4, while it decreased with more stringent end-points. It is possible that certain patient characteristics (prevalent cases with a long disease duration at enrollment and unspecified phase of the disease course i.e. recent or chronic, persistent flare at randomization) and study design (use of glucocorticosteroids and immunosuppressives, stringency of the outcomes) influence the response to placebo.
To cite this abstract in AMA style:
Tselios K, Wakani L, Gladman D, Su J, Urowitz M. Response to Placebo in Randomized Clinical Trials with Biologics in Non-renal, Non-neuropsychiatric Systemic Lupus Erythematosus: A Systematic Review and Pooled Analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/response-to-placebo-in-randomized-clinical-trials-with-biologics-in-non-renal-non-neuropsychiatric-systemic-lupus-erythematosus-a-systematic-review-and-pooled-analysis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/response-to-placebo-in-randomized-clinical-trials-with-biologics-in-non-renal-non-neuropsychiatric-systemic-lupus-erythematosus-a-systematic-review-and-pooled-analysis/