Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Sarilumab, a human mAb against the IL-6 receptor α, has been approved in RA and is being investigated for PMR. The SAPHYR study (NCT03600818) of sarilumab in patients with glucocorticoid (GC) resistant PMR, who flared on ≥7.5 mg/day prednisone or equivalent, met its primary endpoint: a significantly greater proportion of patients receiving sarilumab + 14 week (wk) GC taper achieved sustained remission at wk 52, compared with placebo + 52 wk GC taper (Dasgupta, EULAR 2022). Here, we present data on the number of patients without any PMR signs and symptoms over time, and patients requiring rescue therapy.
Methods: Patients were recruited between Oct 2018 to Jul 2020, and randomized (1:1) to receive sarilumab 200 mg every 2 wks (Q2W) + a faster 14 wk GC tapered regimen (sarilumab arm) OR placebo Q2W + a longer 52 wk GC tapered regimen (comparator arm); treatment duration was 52 wks. After the baseline visit, patients were followed up at wks 2 and 4, and then every 4 wks until wk 24, and then at wks 32, 40, and 52. Patients who experienced a disease flare or could not adhere to per-protocol GC taper stopped GC taper and received rescue GC therapy.
Results: A total of 118 patients of the intended 280 were recruited as the study was prematurely terminated due to protracted recruitment timelines and the COVID-19 pandemic; 117 patients were treated (sarilumab, n=59; comparator, n=58).
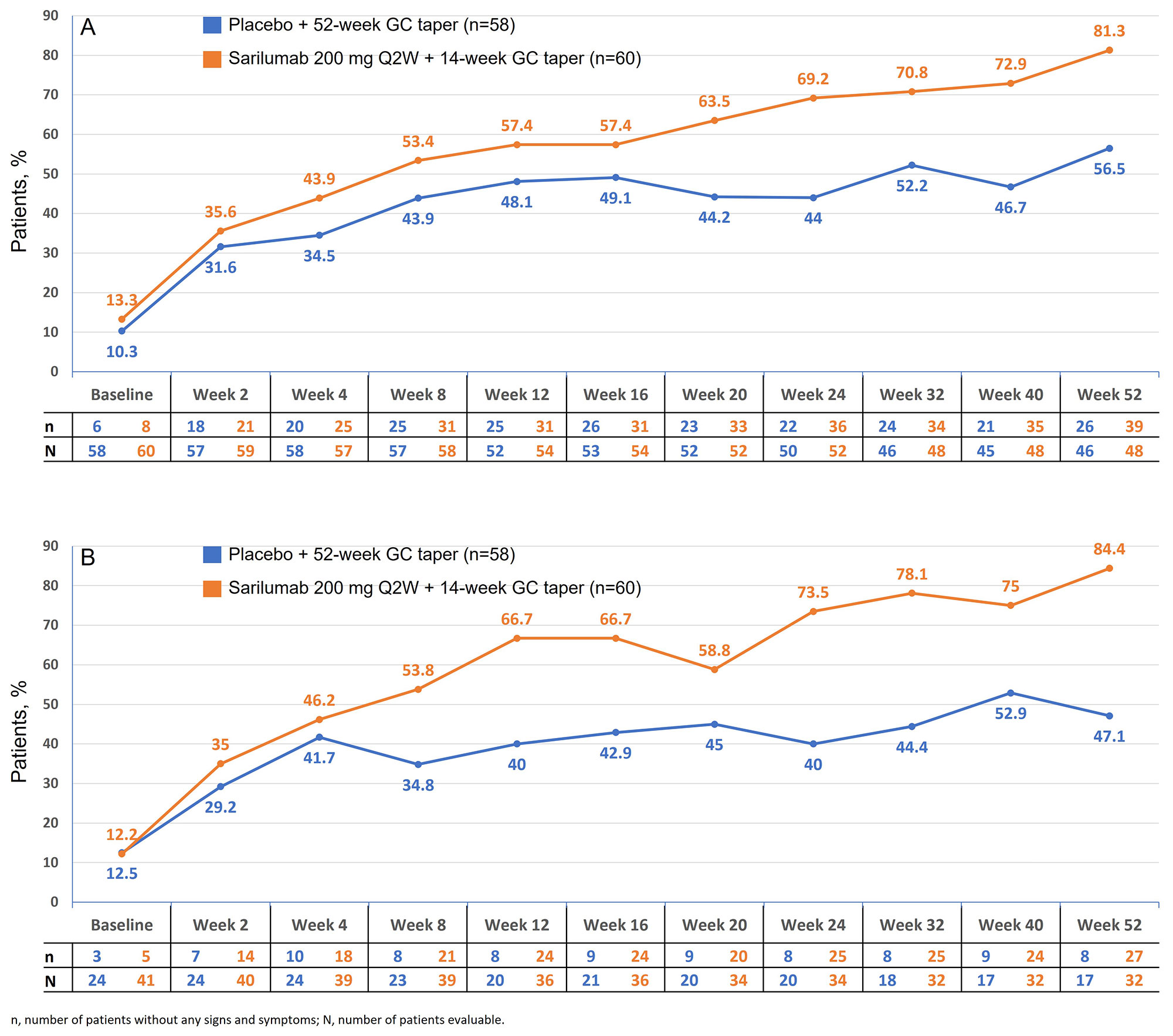
In the sarilumab arm, the proportion of patients without PMR signs and symptoms increased at week 2 and continued to increase over time until week 52. At each visit after baseline, the proportion of patients without any PMR signs and symptoms was higher in the sarilumab arm vs the comparator arm (Figure 1A). A similar trend was observed when patients receiving the rescue therapy were excluded from the analyses (Figure 1B). Patients in the sarilumab arm were less likely to have a flare after achieving clinical remission vs the comparator arm (16.7% vs 29.3%; HR 0.56; 95% CI 0.35–0.90; P=0.0158).
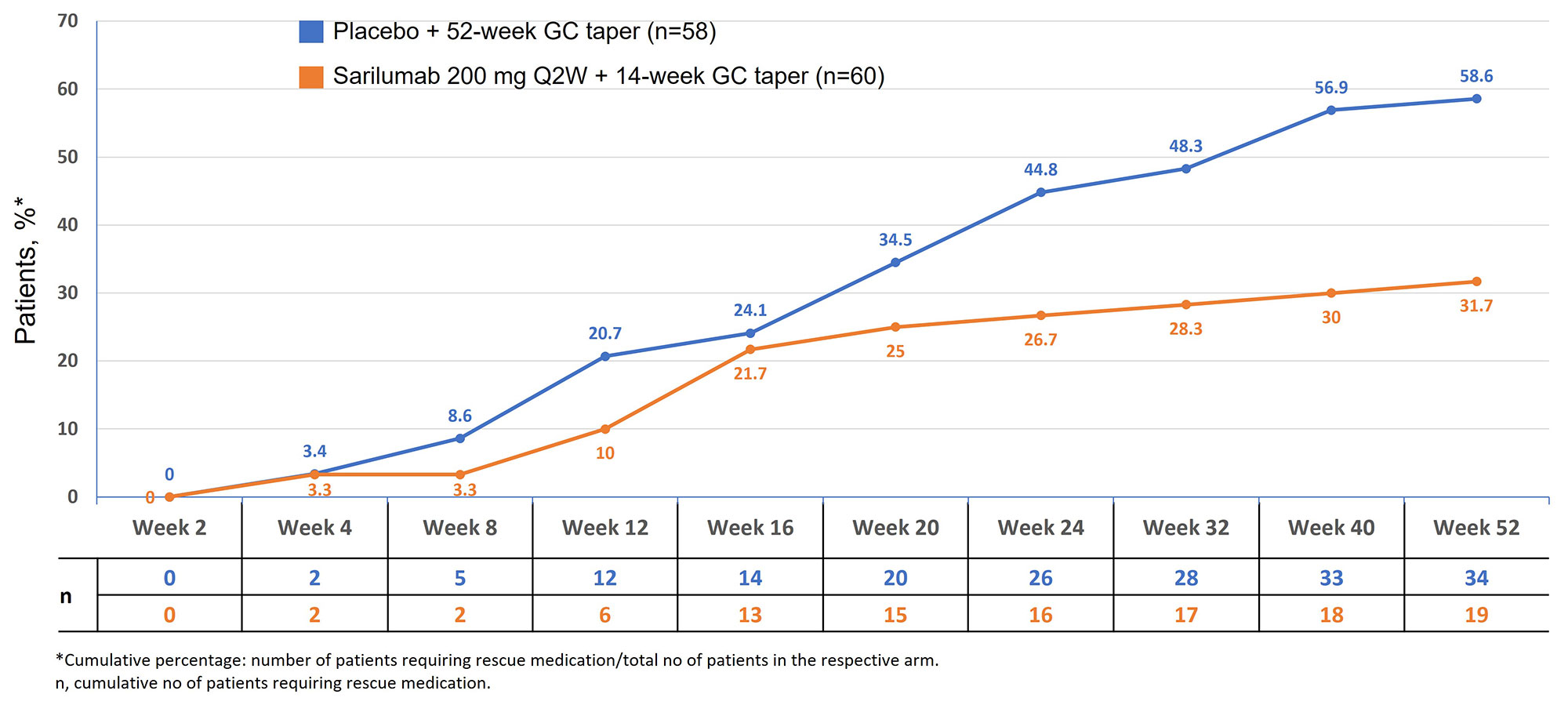
A higher proportion of patients in the comparator arm, vs the sarilumab arm, required additional GC (rescue therapy) during the study period (58.6% vs 32.2% P=0.0053 [Fisher’s exact test]). The cumulative proportion of patients requiring rescue therapy was higher at each timepoint after baseline up to wk 52 in the comparator arm vs the sarilumab arm (Figure 2). In patients receiving rescue GC, the median (range) cumulative rescue GC dose over 52 wks was 1076.1 mg (8–2108) in the sarilumab arm vs 1326.5 mg (20-2484) in the comparator arm (P=0.5078).
There were no new safety signals, and the adverse events were consistent with the known safety profile of sarilumab.
Conclusion: Sarilumab demonstrated efficacy in difficult to treat GC resistant PMR patients, consistent with results observed with another IL-6 receptor inhibitor in new onset PMR (Bonelli, Ann Rheum Dis. 2022). A greater proportion of patients in the sarilumab arm had no PMR signs and symptoms at each timepoint after baseline vs the comparator arm, confirming that sarilumab provided faster relief of PMR activity vs the comparator arm.
To cite this abstract in AMA style:
Spiera R, Unizony S, Warrington K, Sloane Lazar J, Giannelou A, Nivens M, Akinlade B, Wong W, Lin Y, Buttgereit F, Devauchelle V, Rubbert-Roth A, Dasgupta B. Resolution of PMR Signs and Symptoms in Patients Treated with Sarilumab: A Phase 3, Multicenter, Randomized, Double Blind, Placebo Controlled Trial (SAPHYR) in Relapsing PMR [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/resolution-of-pmr-signs-and-symptoms-in-patients-treated-with-sarilumab-a-phase-3-multicenter-randomized-double-blind-placebo-controlled-trial-saphyr-in-relapsing-pmr/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/resolution-of-pmr-signs-and-symptoms-in-patients-treated-with-sarilumab-a-phase-3-multicenter-randomized-double-blind-placebo-controlled-trial-saphyr-in-relapsing-pmr/