Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients with axial spondyloarthritis (axSpA), treatment goals consist of achieving remission or low disease activity (LDA) to alleviate symptoms, improve function, decrease disease complications, and forestall skeletal damage. Notably, it has been shown that failure to achieve Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) < 3 leaves significant disease activity for patients. No studies have yet used patient information from multiple Canadian rheumatology registries to better understand outcomes and unmet needs in patients with axSpA. The objective of this study was to describe residual disease activity in Canadians with axSpA treated with currently available therapies.
Methods: This is a multi-registry, observational, retrospective analysis of data extracted from the Rhumadata™ (Quebec), SPARCC (East/Atlantic and West regions, and Ontario), and FORCAST (Alberta) registries. Data were extracted for up to 12 months of the latest axSpA therapy initiation in adult patients between January 2010 and December 2020. The primary endpoint was the proportion of patients who failed to achieve sustained LDA at 12 months; LDA was defined as a BASDAI score < 3, and sustained LDA defined as achieving LDA at both 6 and 12 months. A secondary endpoint further described the proportion of patients who failed to achieve sustained LDA with a definition of BASDAI score < 4. Analyses included outcomes by treatment class (NSAIDs, TNFi, or IL-17i) and number of prior advanced therapies. Data from each registry and region were analyzed separately using a standard set of inclusion/exclusion criteria and statistical analysis plan.
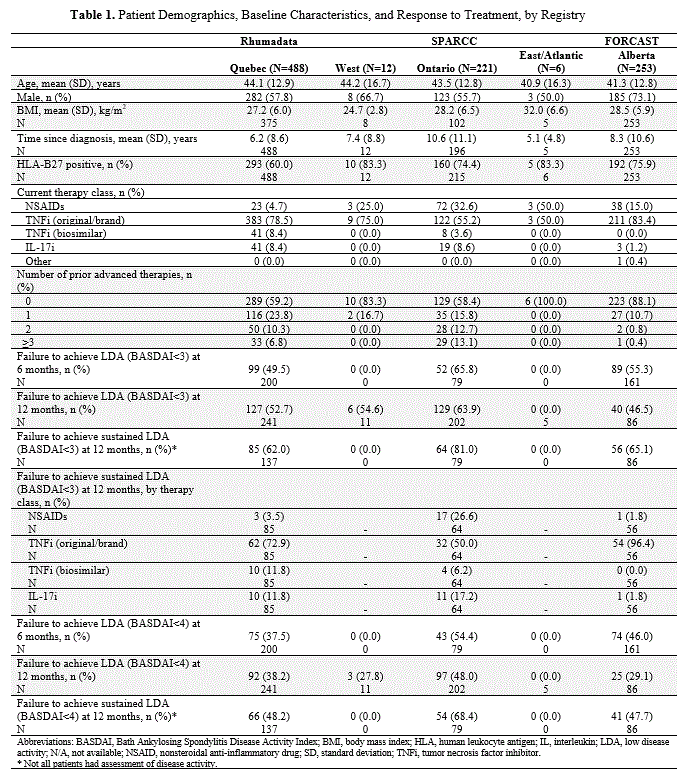
Results: A total of 980 patients (Rhumadata™, N=488; SPARCC, N=239; FORCAST, N=253) were included. The mean age of patients ranged from 40.9 to 44.2 years, with a majority of male and HLA‑B27 positive patients overall (Table 1). Mean time since diagnosis ranged from 5.1 to 10.6 years. In all 3 registries, most patients had failed NSAIDs and were receiving their first advanced therapy. The most common current advanced therapy class used was TNFi (Table 1) with less than 10% of patients receiving IL-17i.Nearly half of all patients from Rhumadata™ and FORCAST and 66% of those from SPARCC failed to achieve LDA (BASDAI < 3) at 6 months. At 12 months, failure to achieve sustained LDA ranged from 62.0% in Rhumadata to 81.0% in SPARCC (Table 1). This trend persisted even when using a less stringent definition to measure sustained LDA (BASDAI < 4). Small sample sizes from the West (n=12) and East/Atlantic (n=6) in SPARCC prevented reliable assessments in these regions.
Conclusion: This analysis demonstrated that most Canadians with axSpA failed to achieve sustained LDA after 12 months of initiating therapy. The variations seen in this analysis may reflect distinct regional patient profiles, differing prior biologic treatments at study entry, different provincial reimbursement criteria, or lack of standardized measurements. There is significant residual disease activity and a high unmet need for improved therapeutic approaches and patient outcomes for Canadians living with axSpA.
To cite this abstract in AMA style:
Choquette D, Maksymowych W, Rahman P, Inman R, Laliberté M, Fournier P, Girard T, Gladman D. Residual Disease Activity in Canadian Patients with Axial Spondyloarthritis: Results from a Multi-registry Analysis (UNISON-Axial SpA) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/residual-disease-activity-in-canadian-patients-with-axial-spondyloarthritis-results-from-a-multi-registry-analysis-unison-axial-spa/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/residual-disease-activity-in-canadian-patients-with-axial-spondyloarthritis-results-from-a-multi-registry-analysis-unison-axial-spa/