Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease with heterogeneous symptoms, complicating diagnosis and treatment. Historically, treatment included broad immunosuppressants, but real-world data now show significant adverse effects associated with traditional treatments. Since 2011, two biologics have been approved for SLE, but clinical trials are not always representative of real-world populations, necessitating post-marketing safety evaluation.
Methods: FDA Adverse Event Reporting System data were analyzed using a disproportionality analysis to detect risk signals for each approved biologic. Reporting odds ratios (ROR) were calculated for each system organ class (SOC) and preferred term (PT), defined by the Medical Dictionary for Regulatory Activities.
Results: In 2023, 125,675 primary suspect adverse events (AEs) were reported among individuals with SLE, including 10,737 belimumab and 661 anifrolumab cases. Belimumab AEs occurred in all 27 SOCs and were significant in five [Social circumstances (ROR 5.27, 95% CI 4.34, 6.39), Reproductive system and breast disorders (ROR: 4.62, 95% CI 2.73, 7.82), Product issues (ROR 3.48, 95% CI 2.96, 4.09), Ear and labyrinth disorders (ROR: 2.26, 95% CI 1.61, 3.17), Respiratory, thoracic and mediastinal disorders (ROR: 2.10, 95% CI 1.90, 2.32)]; and anifrolumab AEs occurred in 25 SOCs and were significant in three [Reproductive system and breast disorders (ROR: 4.36, 95% CI 1.57, 12.06), Renal and urinary disorders (ROR: 2.96, 95% CI 1.93, 4.53), Cardiac disorders (ROR: 2.15, 95% CI 1.35, 3.41)]. In total, 169 belimumab PTs and 20 anifrolumab PTs displayed significant disproportionality. The top five belimumab PTs by strength of safety signal were Product complaint (ROR 164.28, 95% CI 22.72, 1187.69), Product storage error (ROR 133.73, 95% CI 18.42, 970.81), Exposure via skin contact (ROR 127.63, 95% CI 17.56, 927.48), Wrong technique in device usage process (ROR 71.09, 95% CI 22.38, 225.82), and Product supply issue (ROR 69.77, 95% CI 9.42, 516.71); for anifrolumab these were Insurance issue (ROR 78.51, 95% CI 17.54, 351.42), Appendicitis (ROR 39.25, 95% CI 11.05, 139.39), Anaphylactic reaction (ROR 31.57, 95% CI 13.34, 74.69), Herpes zoster (ROR 23.15, 95% CI 14.23, 37.65), and Cardiac failure (ROR 19.65, 95% CI 7.12, 54.21).
Conclusion: In belimumab clinical trials, the most common SOC AEs were infections, gastrointestinal, musculoskeletal, nervous system, and skin disorders. Each of these was frequent in this study, but not disproportionately high. However, five other SOCs show significant disproportionality, indicating possible safety concerns not identified in trials. In anifrolumab clinical trials, the most common SOC AEs were infections, general and administration site conditions, respiratory disorders, and musculoskeletal disorders. None showed disproportionately high safety signals in this study, but three additional SOCs were disproportionately high.Despite limitations of spontaneous reporting systems, these findings highlight potential safety concerns with biologics in SLE. Further research using real-world data is needed.
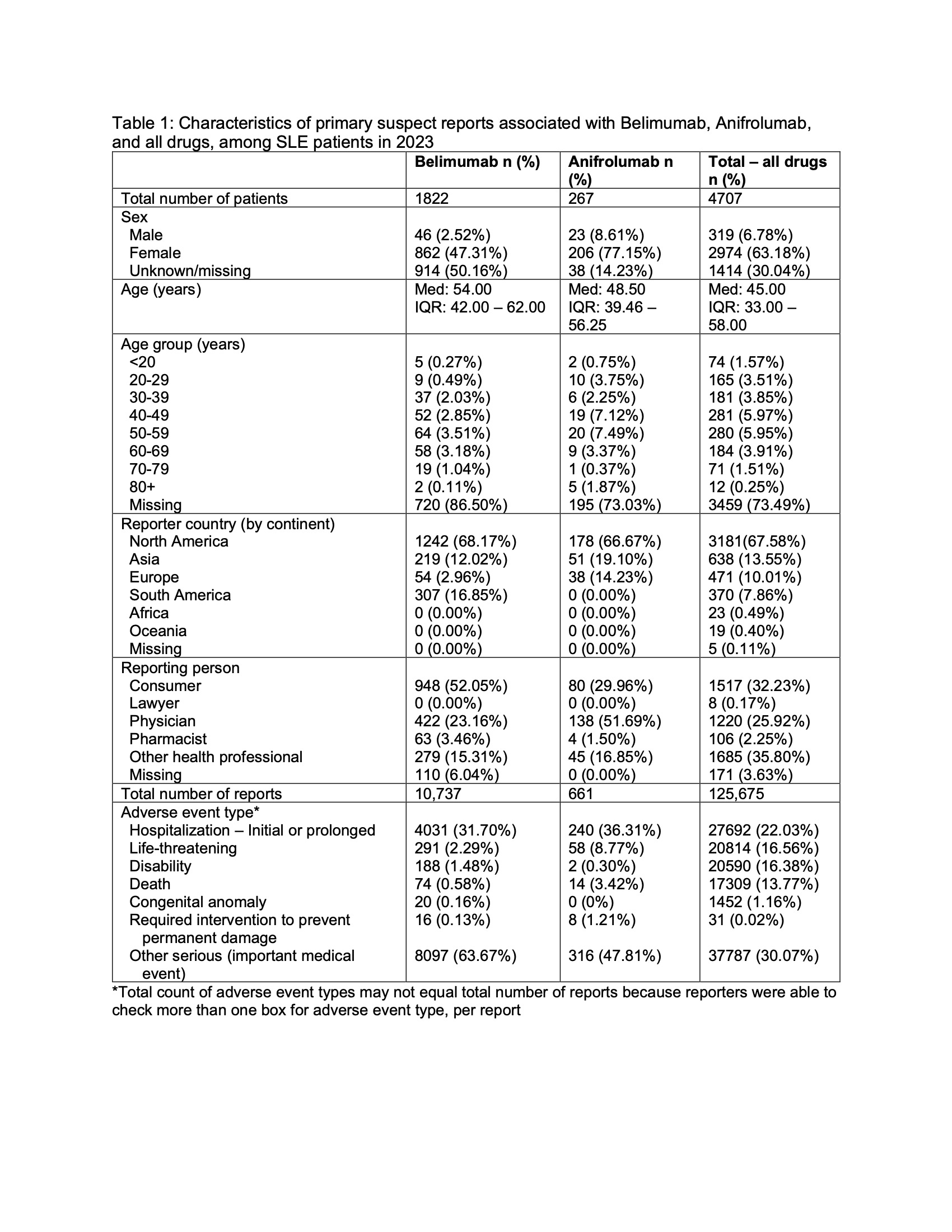 Table 1: Characteristics of primary suspect reports associated with belimumab, anifrolumab, and all drugs, among SLE patients in 2023
Table 1: Characteristics of primary suspect reports associated with belimumab, anifrolumab, and all drugs, among SLE patients in 2023
.jpg) Table 2a: Signal strength of reports of belimumab among SLE patients at the System Organ Class (SOC) level
Table 2a: Signal strength of reports of belimumab among SLE patients at the System Organ Class (SOC) level
.jpg) Table 2b: Signal strength of reports of anifrolumab among SLE patients at the System Organ Class (SOC) level
Table 2b: Signal strength of reports of anifrolumab among SLE patients at the System Organ Class (SOC) level
To cite this abstract in AMA style:
Crisci S. Reported Adverse Events Associated With Systemic Lupus Erythematosus (SLE) Treatment: Insights From the FDA Adverse Event Reporting System (FAERS) 2023 [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/reported-adverse-events-associated-with-systemic-lupus-erythematosus-sle-treatment-insights-from-the-fda-adverse-event-reporting-system-faers-2023/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reported-adverse-events-associated-with-systemic-lupus-erythematosus-sle-treatment-insights-from-the-fda-adverse-event-reporting-system-faers-2023/
