Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Extended observations in clinical trials have not demonstrated an increased risk of serious infection events (SIE) in patients with rheumatoid arthritis (RA) treated with rituximab.1 However, continuous surveillance using large-scale observational data is of importance. Therefore, the objective of this study was to evaluate the rate of SIEs among patients with RA who received only an initial rituximab infusion vs those retreated with ≥ 1 rituximab infusion during the first year of therapy, and also to describe characteristics of rituximab-treated patients who experienced an SIE vs those who did not.
Methods: Patients with RA enrolled in the Corrona registry (NCT01402661) and treated with rituximab were followed until their most recent Corrona registry visit, first SIE, switch to another biologic or targeted synthetic disease-modifying antirheumatic drug, or 12 months after the most recent infusion with no further retreatment – whichever occurred first. The rate of SIEs was estimated in the overall population as well as in patients retreated with ≥ 1 infusion every 12 months after rituximab initiation and in those who did not receive a repeat infusion in the first 12 months. Patient characteristics were compared between those who experienced an SIE and those who did not.
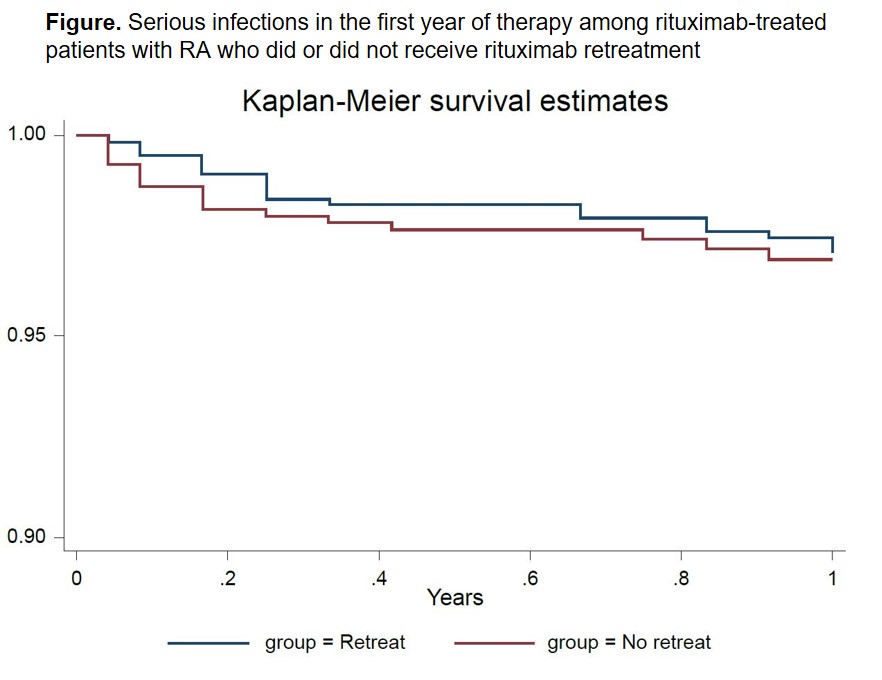
Results: A total of 1361 patients with 1821 patient-years (PY) of follow-up were included; there were 59 SIEs for a rate of 3.24 SIE/100 PYs. 637 patients (46.8%) received ≥ 1 rituximab retreatment during the first 12 months and 724 (53.2%) received only the initial infusion. In the retreatment population there were 40 SIEs per 1312.8 PY for a rate (95% CI) of 3.05/100 PY (2.18-4.15), and in the no retreatment population there were 19 SIEs per 508.71 PY for a rate (95% CI) of 3.73/100 PY (2.25-5.83). The Kaplan-Meier curve depicting the occurrence of SIEs in the 2 cohorts during the first year of follow-up is shown (Figure). In the 59 patients (4.3%) who experienced an SIE, the mean (SD) number of rituximab infusions was 1.88 (1.18), compared with 2.07 (1.70) in the 1302 patients (95.7%) who did not experience an SIE. Patients who experienced an SIE vs those who did not were older (mean age [SD]: 62.9 [9.9] vs 58.1 [12.55] years), had longer disease duration (19.1 [13.1] vs 13.6 [10.4] years), were more frequently diabetic (16.9% vs 8.3%) and more frequently had cardiovascular disease (25.4% vs 12.8%), prior history of SIEs (18.6% vs 5.8%) and pulmonary disease (10.2% vs 4.8%). There were no differences in other clinical, demographic and medication history characteristics; steroid therapy was similar between the groups.
Conclusion: Retreatment with rituximab infusions did not result in a higher rate of SIEs in this study. Patients who experienced an SIE had a higher prevalence of risk factors for infections.
References:
1. van Vollenhoven RF, et al. J Rheumatol. 2015;42:1791-6.
To cite this abstract in AMA style:
Pappas DA, Reed GW, Zlotnick S, Best J, Magner R, Persuitte G, Greenberg JD. Repeated Rituximab Infusions for the Therapy of Rheumatoid Arthritis Is Not Associated with Increased Rates of Serious Infections [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/repeated-rituximab-infusions-for-the-therapy-of-rheumatoid-arthritis-is-not-associated-with-increased-rates-of-serious-infections/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/repeated-rituximab-infusions-for-the-therapy-of-rheumatoid-arthritis-is-not-associated-with-increased-rates-of-serious-infections/