Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate (MTX) is often used as first-line DMARD therapy in active psoriatic arthritis (PsA). The value of MTX in combination with different bDMARDs is still unclear. We designed an investigator-initiated, randomized, placebo-controlled trial (IIT) in active PsA to examine if outcomes of treatment with ustekinumab (UST) in combination with MTX (either newly initiated or ongoing) were different from UST only (+Placebo; PBO). It was allowed to include patients with stable MTX treatment. Here, we analyze the data stratified to MTX pretreatment, focusing on early treatment response within the first 16 weeks.
Methods: A total of 186 patients with active PsA (defined as TJC≥4, SJC≥4 [68/66 joint count], and DAS28≥3.2) were screened for eligibility. 173 patients were randomized to UST+MTX (new or ongoing) or UST+PBO. Patients were stratified regarding their previous MTX therapy (blinded continuation of MTX or replacement of MTX with PBO [MTX-pre-treated] or blinded newly initiated MTX or PBO [MTX-naïve]). Demographic data and disease activity status on peripheral arthritis (response rates for LDA and remission for DAS28-CRP, DAPSA) were compared between treatment groups regarding MTX pretreatment. Early response behavior was investigated by identification of DAS28-CRP and DAPSA low disease activity (LDA) and remission rates at weeks 4, 16, and 24.
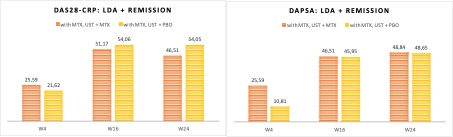
Results: BL data were well-balanced between treatment groups (UST+MTX, n=87; UST+PBO, n=79), including gender (42.5% vs. 40.5% female) and mean values for age (49.2 vs. 47.2 years), BMI (29.4 vs. 28.9 kg/m2), SJC (8 vs. 8), TJC (12 v.s 12), DAS28-CRP (4.6 v.s 4.4), DAPSA (36.7 v.s 34.9), PASI (2.8 .vs 2.4), enthesitis (LEI >0: 50.57% .vs 50.63%), and other domains. BL differences were seen in dactylitis (24.1% .vs 19.0%), BSA (2.9%. vs. 1.0%), and DLQI (8.6. vs 6.9). The patient cohort stratified according to MTX pretreatment was taken out (n=80) for analysis. The proportion of patients who reached LDA and remission rates in DAS28-CRP was slightly lower in the group who discontinued MTX treatment at the study start compared to those who continued MTX (DAS28-CRP 21.62% vs. 25.59%, respectively). This was more prominent in analyzing the achievement of LDA/remission rates in DAPSA at week 4 (10.81% for those discontinuing MTX vs. 25.59 % for those who continued MTX (Figure 1)). At weeks 16 and 24, both treatment groups reached the same levels for LDA/remission rates in DAS28-CRP and DAPSA.
Conclusion: We have previously shown that MTX+UST vs. UST+PBO is non-inferior for efficacy assessments in active PsA. Here we present the data from the analysis focusing on early response behavior to answer whether the overlapping treatment with MTX is of clinical value after the decision to initiate a biological therapy with ustekinumab is made. With a focus on DAS28-CRP and DAPSA response rates (LDA+remission), we can show that an overlapping treatment by the continuation of MTX within the first 12-16 weeks is of clinical value to increase response rates in the early treatment phase of UST treatment. MTX can be stopped at week 12 when UST efficacy increases, independent of MTX use.
To cite this abstract in AMA style:
Koehm M, Foldenauer A, Rossmanith T, Kellner H, Kiltz U, Kleyer A, Burmester G, Kofler D, Brandt-Juergens J, Bergner R, Behrens F. Removal of Methotrexate in Patients with Active Psoriatic Arthritis with Newly Induced Ustekinumab Treatment Leads to a Delayed Response in DAPSA and DAS28 Within the First 16 Weeks [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/removal-of-methotrexate-in-patients-with-active-psoriatic-arthritis-with-newly-induced-ustekinumab-treatment-leads-to-a-delayed-response-in-dapsa-and-das28-within-the-first-16-weeks/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/removal-of-methotrexate-in-patients-with-active-psoriatic-arthritis-with-newly-induced-ustekinumab-treatment-leads-to-a-delayed-response-in-dapsa-and-das28-within-the-first-16-weeks/