Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The link between treatment recommendations for PsA (to aim for a state of remission or low disease activity (LDA))1,2 and patient-important outcomes has been little explored. The objective of this analysis was to investigate the potential of remission or LDA according to the clinical Disease Activity Index for PsA (cDAPSA) and achievement of very low/minimal disease activity (VLDA/MDA) during treatment with ustekinumab (UST) or TNF inhibitor (TNFi), as well as the association between these outcomes and health-related quality of life (HRQoL).
Methods: PsABio (NCT02627768) is an ongoing real-world observational study in eight European countries where PsA patients receive 1st-, 2nd- or 3rd-line biologics (either UST or TNFi). Of 563 UST- or TNFi-treated patients enrolled Dec 2015 – Aug 2017, 303 had data available at 6 months and were analyzed here. Disease states were defined using cDAPSA ≤4 for remission and ≤13 for LDA (data available for 250 patients) and VLDA 7/7 and MDA 5/7 criteria (data available data for 206 and 260 patients, respectively), and HRQoL using EQ5D (data available for 249 patients with MDA availability). Available observed data are presented, with no imputation of missing data.
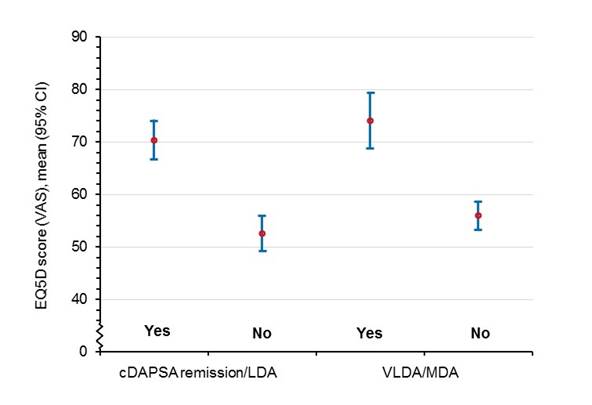
Results: For the 303 patients analyzed, mean age was 49.7 (standard deviation, SD 12.8) years, mean disease duration was 7.2 (SD 8.2) years, and 50.5% were women. The table shows data at 6 months for cDAPSA remission, cDAPSA LDA, VLDA, and MDA in UST- and TNFi-treated patients. cDAPSA remission/LDA and VLDA/MDA achievement (Yes vs No) were associated with better HRQoL based on mean (95% confidence interval, CI) EQ5D visual analog scale (VAS) at 6 months, as shown by non-overlapping CIs in the figure. Assessment of Psoriasis Skin Disease (68/299=22.7%) was the most frequently missed MDA component, while enthesitis was the least frequently missed (6/299=2.0%). The other five components were all missed with equal frequency (8–9%).
|
|
UST-treated patients n/N (%) |
TNFi-treated patients n/N (%) |
|
cDAPSA remission (cDAPSA ≤4) |
25/126 (19.8) |
25/124 (20.2) |
|
cDAPSA LDA (including remission) (cDAPSA ≤13) |
64/126 (50.8) |
66/124 (53.2) |
|
VLDA |
11/102 (10.8) |
12/104 (11.5) |
|
MDA (including VLDA) |
38/132 (28.8) |
38/128 (29.7) |
Conclusion: Remission and/or LDA appeared to be reachable outcomes in this real-world study, since among patients treated with UST or TNFi for 6 months, cDAPSA remission/LDA was achieved by approx. 50% of patients, and VLDA/MDA by approx. 30% of patients, irrespective of the type of therapy. Furthermore, these disease states were associated with improved HRQoL, making these outcomes patient-relevant.
1. Gossec L, et al. Ann Rheum Dis. 2016;75(3):499–510.
2. Smolen JS, et al. Ann Rheum Dis. 2017;77(1):3–17.
To cite this abstract in AMA style:
Gossec L, Bergmans P, de Vlam K, Gremese E, Joven BE, Korotaeva T, Nurmohamed M, Sfikakis P, Siebert S, Smirnov P, Theander E, Smolen JS. Remission/Low Disease Activity Is a Reasonable Treatment Target in Psa: Results from a Routine Care European Cohort of Psa Patients Treated with Ustekinumab or TNF Inhibitors [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/remission-low-disease-activity-is-a-reasonable-treatment-target-in-psa-results-from-a-routine-care-european-cohort-of-psa-patients-treated-with-ustekinumab-or-tnf-inhibitors/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/remission-low-disease-activity-is-a-reasonable-treatment-target-in-psa-results-from-a-routine-care-european-cohort-of-psa-patients-treated-with-ustekinumab-or-tnf-inhibitors/