Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Patient (pt)-reported pain is common in rheumatoid arthritis (RA), even in pts with good disease control1. This analysis evaluated pain control achieved by methotrexate (MTX), baricitinib (BARI), or combination of baricitinib+MTX (BARI+MTX) in DMARD-naive RA pts from a Phase 3 study, RA-BEGIN2.
Methods:
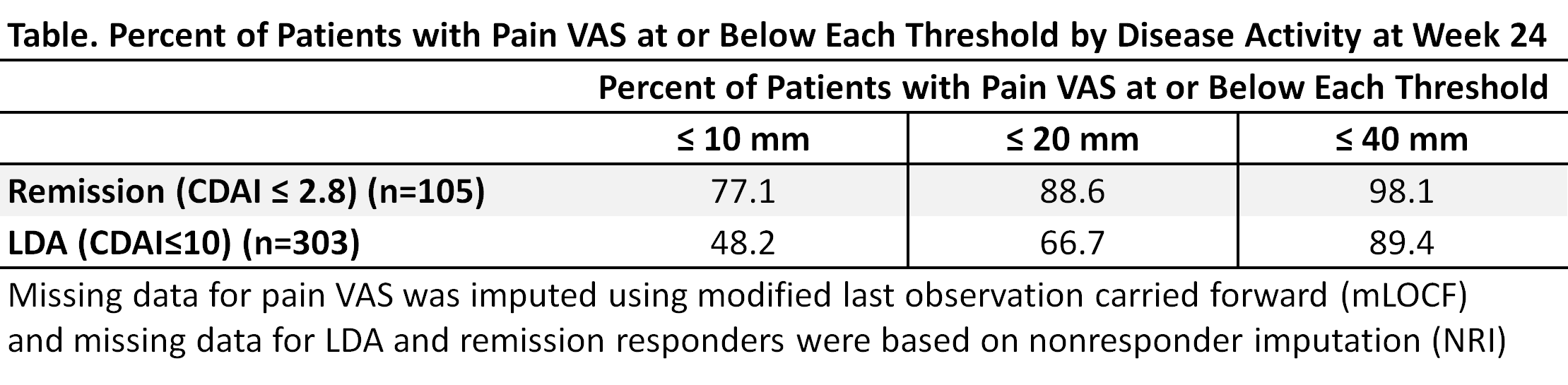
Pts were randomized and treated with MTX (N=210), BARI (4 mg QD, N=159), or BARI+MTX (N=215). Pain intensity was assessed on a 0-100 mm visual-analog scale (VAS). Percentage of pts reporting improvement in pain of ≥30%, ≥50%, and ≥70% was evaluated, and time to achieve these improvements was assessed with the cumulative incidence estimate3. Between-group comparisons of pain improvement (≥30%, ≥50%, and ≥70%) and percentage achieving low disease activity (LDA) and remission were made using logistic models. Pain improvement among pts achieving remission (CDAI ≤2.8) or LDA (CDAI ≤10) was assessed relative to thresholds for ‘remaining’ pain (pain VAS ≤10 mm, ≤20 mm, or ≤40 mm) at Week 24.
Results:
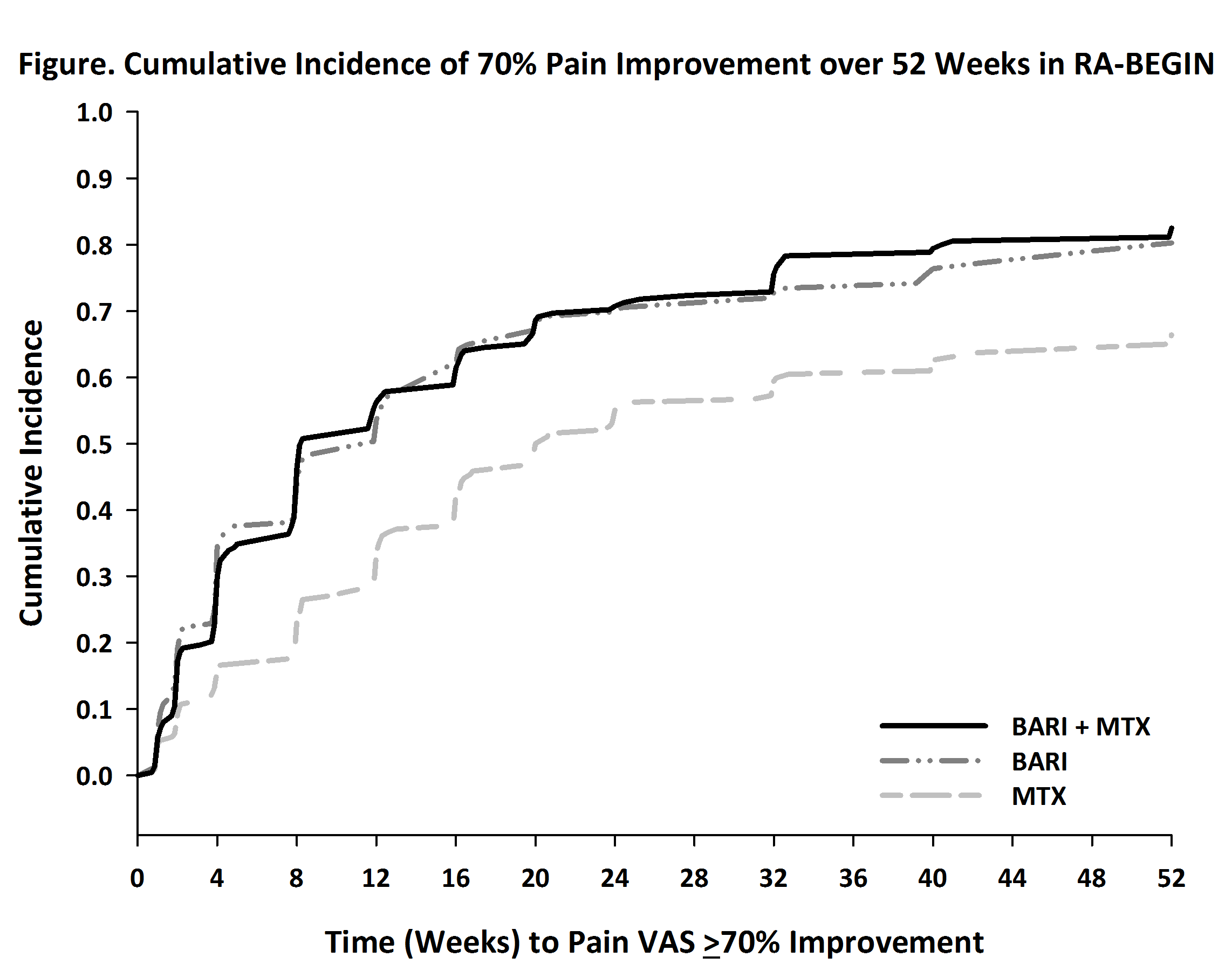
The mean (SD) baseline pain VAS for MTX, BARI, and BARI+MTX were 65.2 (24.1), 64.1 (21.6), and 62.6 (22.6), which improved to 35.2 (26.2), 24.2 (22.2), and 23.8 (23.0) at Week 24, respectively. BARI and BARI+MTX resulted in more pain improvement (-40.8 and -41.2, respectively, p<0.001) than MTX (-30.0). The percentage of pts reaching ≥70% pain improvement by Week 24 was 32.7% for MTX, 50.0% for BARI (p≤0.001 vs. MTX), and 50.5% for BARI+MTX (p≤0.001 vs. MTX). The time when 50% of pts achieved 70% pain improvement was 20 weeks for MTX, 12 weeks for BARI and 8 weeks for BARI+MTX, respectively. The cumulative incidence for achieving 70% improvement is shown (Fig). BARI and BARI+MTX demonstrated similar pain improvement responses. The percentage of pts achieving remission at Week 24 was higher for BARI (21%) and BARI+MTX (22%) compared to MTX (11%)2. Similarly, the percentage of pts achieving LDA was higher for BARI (60%) and BARI+MTX (59%) compared to MTX (39%).2 Mean pain VAS at Week 24 was 6.9 and 16.8, respectively, for pooled patients in remission and LDA. Across treatment groups, the percentage of pts achieving ≤10, ≤20 or ≤40 mm remaining pain by remission and LDA are presented in the Table.
Conclusion:
More pts treated with BARI or BARI+MTX had greater levels of pain improvement more rapidly compared to MTX. Pts treated with BARI or BARI+MTX were more likely to achieve remission and LDA. Patients achieving remission had less remaining pain than those in LDA.
References:
1 Altawil, et al Arthritis Care Res 2016
2 Fleischmann, et al Arthritis Rheumatol 2017
3 Gooley TA Statist Med 1999
To cite this abstract in AMA style:
Lee YC, Emery P, Bradley JD, Zhu B, Gaich CL, Cai Z, Quebe A, Cardoso A, Chen YF, Fleischmann R. Remaining Pain in DMARD-Naive Rheumatoid Arthritis Patients Treated with Baricitinib and Methotrexate [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/remaining-pain-in-dmard-naive-rheumatoid-arthritis-patients-treated-with-baricitinib-and-methotrexate/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/remaining-pain-in-dmard-naive-rheumatoid-arthritis-patients-treated-with-baricitinib-and-methotrexate/