Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a complex heterogeneous disease associated with multiple domains. Psoriatic Arthritis Disease Activity Score (PASDAS) is a composite disease activity measure for PsA, developed to assess improvements in multiple clinical outcomes in response to treatment using a single instrument.1 The objective of this post hoc analysis was to evaluate associations between PASDAS and a set of patient-reported outcomes (PROs).
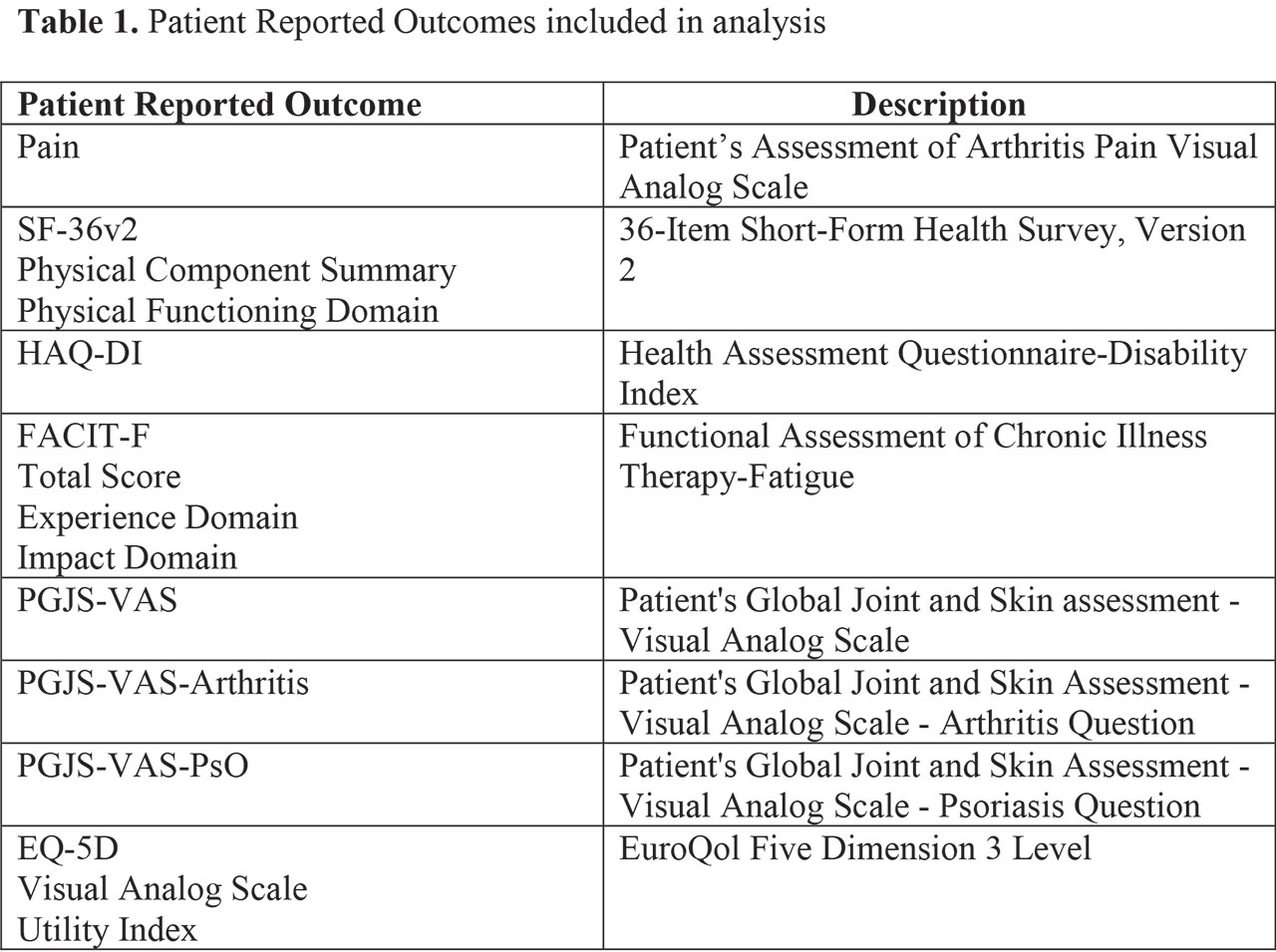
Methods: All available data from two Phase 3 studies in patients with PsA (OPAL Broaden [12 months; NCT01877668]; OPAL Beyond [6 months; NCT01882439])2,3 were analyzed. PASDAS (score range 0–10) is based on the following measures: Patient’s Global Assessment of PsA Visual Analog Scale (VAS); Physician’s Global Assessment of PsA VAS; tender and swollen joint counts; Leeds Enthesitis count; tender dactylitis count; Short-Form 36 Health Survey Physical Component Summary (SF-36 PCS); and C-reactive protein. PASDAS cut-offs for low and high disease activity are 3.2 and 5.4, respectively.4 A set of PROs was analyzed (Table 1). A repeated measures regression model was used to evaluate the relationship between PASDAS and PROs. A sensitivity analysis to assess the linearity assumption was performed.
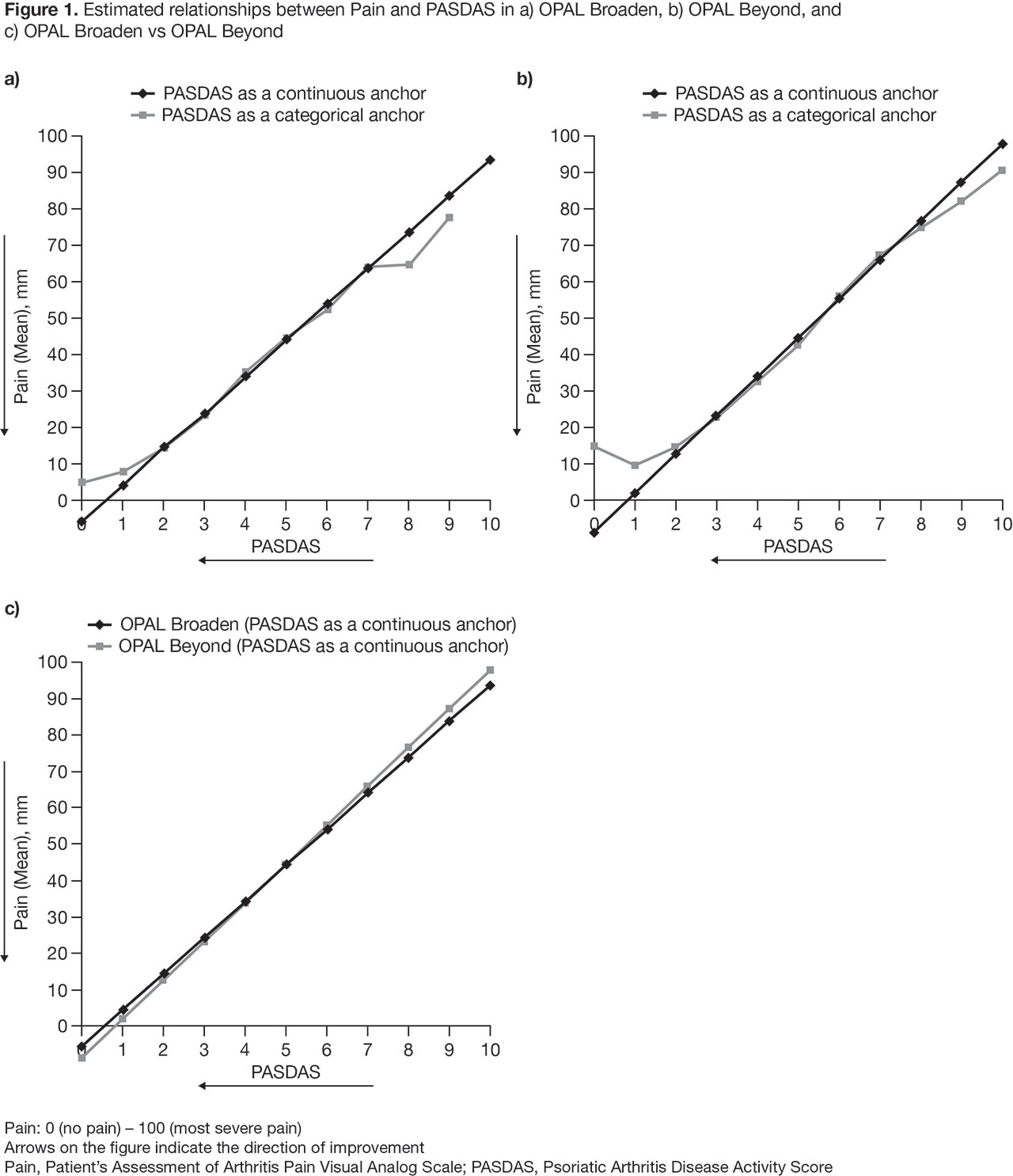
Results: An approximately linear relationship was observed between PASDAS and PROs, which was consistent across all PROs and both studies. For example, in OPAL Broaden, a 1‑point difference in PASDAS was associated with a mean (95% CI) difference in Patient’s Assessment of Arthritis Pain VAS (Pain) score of 9.9 mm (9.4, 10.4) (OPAL Beyond: 10.7 mm [10.1, 11.2]). Mean Pain scores (95% CI) corresponding to PASDAS low and high disease activity cut-offs, respectively, were 26.3 mm (25.0, 27.5) and 48.0 mm (46.7, 49.4) in OPAL Broaden (OPAL Beyond: 25.4 mm [23.7, 27.1] and 48.8 mm [47.4, 50.3], respectively) (Figure 1). In OPAL Broaden, a 1‑point difference in PASDAS was associated with a mean (95% CI) difference in Patient’s Global Joint and Skin assessment (PGJS)-VAS score of 12.0 mm (11.6, 12.4) (OPAL Beyond: 12.6 mm [12.1, 13.1]). Mean (95% CI) PGJS scores corresponding to PASDAS low and high disease activity cut-offs, respectively, were 28.2 mm (27.1, 29.3) and 54.7 mm (53.6, 55.8) in OPAL Broaden (OPAL Beyond: 27.8 mm [26.3, 29.2] and 55.4 mm [54.1, 56.7], respectively).
Conclusion: The approximately linear association observed here provides insight into the relationship between PASDAS and examined PROs, some of which were components of PASDAS, while others were independent outcomes. Results were similar in both studies, suggesting that findings are consistent across different populations.
- Helliwell PS et al. Ann Rheum Dis 2013;72:986–91.
- Gladman D et al. N Engl J Med 2017;377:1525–36.
- Mease P et al. N Engl J Med 2017;377:1537–50.
- Helliwell PS et al. J Rheumatol 2014;41:1212–17.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Jennifer Higginson of CMC Connect and funded by Pfizer Inc.
To cite this abstract in AMA style:
Coates L, Bushmakin A, FitzGerald O, Gladman D, Fallon L, Cappelleri J, Hsu M, Helliwell P. Relationships Between Psoriatic Arthritis Disease Activity Score and Patient-Reported Outcomes in Patients with Psoriatic Arthritis: Post Hoc Analysis of Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/relationships-between-psoriatic-arthritis-disease-activity-score-and-patient-reported-outcomes-in-patients-with-psoriatic-arthritis-post-hoc-analysis-of-two-phase-3-studies/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relationships-between-psoriatic-arthritis-disease-activity-score-and-patient-reported-outcomes-in-patients-with-psoriatic-arthritis-post-hoc-analysis-of-two-phase-3-studies/