Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: RA patients typically present with pain and morning stiffness (MS). MS is predictive of both functional disability and escalated RA care, but the best way to evaluate MS in RA has yet to be determined. Data evaluating MS duration (MSD), MS severity (MSS), and pain intensity (PI) upon awakening with other RA measures representing disease progression and response would help to determine if one is more clearly related to disease activity. Chronotherapy with a modified (delayed)-release (MR) prednisone tablet given at bedtime has shown MS reduction compared to conventional, immediate-release (IR) prednisone with a sustainable effect up to 12 months. Data from the Circadian Administration of Prednisone in Rheumatoid Arthritis-2 (CAPRA-2) study in patients with active RA and on stable DMARD therapy provide the opportunity to evaluate possible relationships between measures of MS and PI with ACR20, DAS28 and HAQ-DI response criteria.
Methods: CAPRA-2 was a 12-week, double-blind, PBO-controlled study that randomized 350 RA patients to 5 mg MR-prednisone (n=231) or PBO (n=119) taken once daily at bedtime (eg, 10pm) in addition to their standard DMARD treatment (Buttgereit, ARD 2012). The primary endpoint was the proportion of patients achieving an ACR20 response after 12 weeks. A key secondary objective was reduction of MS at week 12, as measured in patient diaries. Pearson Correlations were performed to evaluate the relationships between change from baseline in MSD (minutes), MSS (VAS) or PI with DAS28 and HAQ-DI. Furthermore a Wilcoxon rank sum analysis was completed between responders and nonresponders based on ACR20, DAS28 (score < 2.6) and HAQ-DI (% change from baseline < -0.22) and MSD.
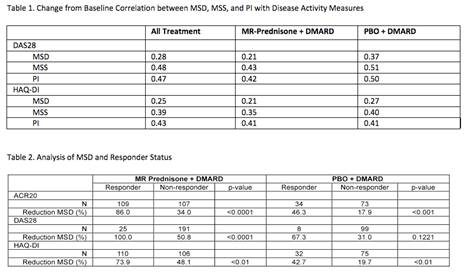
Results: MSD, MSS and PI showed correlation with DAS28 and HAQ-DI in all group analyses (p<0.0001). Stronger absolute correlations were seen with MSS and PI than with MSD, whether the study groups were analyzed separately or together (all treatment, Table 1). Specifically, a moderately strong correlation (≥ 0.5) was seen between DAS28 and MSS and PI in the treatment and PBO groups. MSS and PI were strongly correlated (0.9). The ranges of correlations found are similar to previous studies showing joint impairment is moderately correlated with disability (0.42-0.50) as measured by self-report questionnaires (Yazici, J Rheumatol 2004). Responders had a greater relative reduction in MSD than non-responders, especially the MR-prednisone patients (Table 2).
Conclusion: MSD, MSS and PI are correlated with DAS28 and HAQ-DI in placebo and MR-prednisone treated patients on DMARDs, with stronger correlations seen with MSS and PI. Patients meeting ACR20, DAS28 and HAQ-DI response criteria had a significantly greater reduction in MS than non-responders. Morning pain and stiffness severity in addition to duration of morning stiffness are key patient reported outcomes for both treatment response and disease progression in RA patients.
Disclosure:
F. Buttgereit,
Merck Serono, Horizon Pharma formerly Nitec Pharma, Mundipharma Int. Ltd.,
5,
Merck Serono, Horizon Pharma (formerly Nitec Pharma) ,
2;
J. R. Kirwan,
Horizon Pharma (formerly Nitec Pharma), AstraZeneca, CombinatoRx, GlaxoSmithKline, Merck, and Wyeth,
5;
K. G. Saag,
Amgen,
2,
Eli Lilly and Company,
2,
Merck Pharmaceuticals,
2,
Novartis Pharmaceutical Corporation,
2,
Amgen,
5,
Eli Lilly and Company,
5,
Merck Pharmaceuticals,
5,
Novartis Pharmaceutical Corporation,
5,
Horizon Pharma (formerly Nitec Pharma,
5;
R. Alten,
Merck Serono, Horizon Pharma (formerly Nitec Pharma),
5,
Merck Serono,
9;
A. Grahn,
Horizon Pharma (formerly Nitec Pharma),
3;
P. Rice,
CliniRx Research,
3;
M. Boers,
Augurex, Bristol-Myers Squibb, CombinatoRx, GlaxoSmithKline, Medimmune, Horizon Pharma (formerlyNitec Pharma), Mundipharma and Roche,
5,
Genentech, Novartis and Sanofi ,
9,
Schering-Plough, UCB,
9.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relationship-between-morning-stiffness-duration-and-severity-pain-intensity-and-measures-of-disease-activity-in-a-12-week-efficacy-study-of-a-modified-delayed-release-prednisone-plus-disease-modif/