Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: During the first 3 years of denosumab treatment in FREEDOM, there were continued increases in bone mineral density (BMD) and a robust reduction in fracture risk (Cummings et al., NEJM 2009). The changes in total hip BMD explained a considerable proportion of the reduction in new or worsening vertebral and nonvertebral fracture risk (Austin et al., JBMR 2011). Here, we conducted a BMD responder analysis and explored if the progressive BMD gains with 6 years of denosumab therapy continued to relate to the observed fracture incidence.
Methods: The long-term efficacy and safety of denosumab for up to 10 years is being investigated in the open-label extension of the 3-year FREEDOM trial. During the extension, all participants receive 60 mg denosumab every 6 months. For the analyses presented here, women from the FREEDOM denosumab group received 3 more years of denosumab for a total of 6 years. The percentages of women treated with denosumab who achieved BMD increases from FREEDOM baseline at the lumbar spine, total hip, and femoral neck were determined. A logistic regression model was used to examine the relationship between change in total hip BMD and new or worsening vertebral fracture. A comparable approach was employed for nonvertebral fracture using the Cox proportional hazards model.
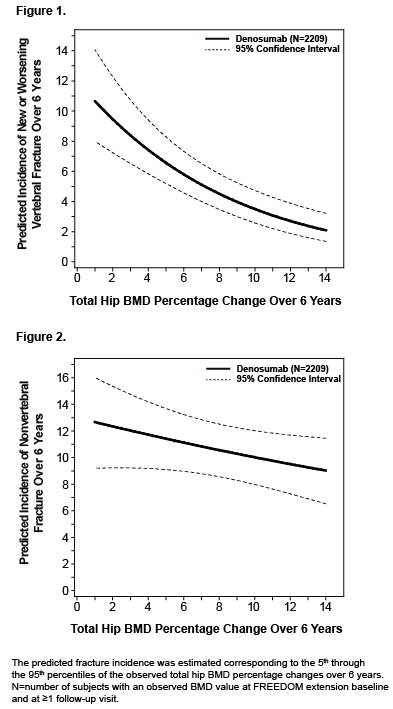
Results: For women who received 3 additional years of denosumab treatment (N=2343 enrolled), further significant increases in BMD occurred for cumulative 6‑year mean gains of 15.2% (lumbar spine), 7.5% (total hip), and 6.7% (femoral neck). At year 6, almost all women treated with denosumab had gains in BMD at the lumbar spine (98%), total hip (96%), and femoral neck (91%). Additionally, 99% of women had gains in BMD at any of these sites, and of these, the gains were >3% in 98% of women and >6% in 95% of women. Fracture incidence remained low during the extension. The relationships between total hip BMD gains and new or worsening vertebral and nonvertebral fractures with 6 years of denosumab treatment are shown in Figures 1 and 2, respectively.
Conclusion: Almost all women who received 6 years of denosumab treatment had gains in BMD at the lumbar spine, total hip, or femoral neck; and those gains were >6% in 95% of them. While on denosumab treatment, the risk of new or worsening vertebral fracture and nonvertebral fracture decreased with increasing percentage change in total hip BMD over 6 years. This association provides clinical relevance to the progressive and continued BMD gains reported with denosumab over time.
Disclosure:
M. A. Bolognese,
Lilly, Amgen, Merck,
2,
Lilly, Amgen, Warner-Chilcott,
5;
P. D. Miller,
Procter and Gamble, Sanofi/Aventis, Roche, Eli Lilly, Merck, Novartis, Amgen, Takeda, Radius, GE,
2,
Warner Chilcott, Merck, Eli Lilly, Amgen, Novartis, Roche, GSK, Baxter, Wright,
5,
Warner Chilcott, Amgen, Novartis, Roche,
8;
J. Y. Reginster,
Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GSK, Roche, Merckle, Nycomed, NPS, Theramex, UCB,
5,
Merck Sharp and Dohme, Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GSK, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Theramex, Nycomed, Novo-Nordisk, Nolver,
9,
Bristol-Myers Squibb, Merck Sharp and Dohme, Rottapharm, Teva, Lilly, Novartis, Roche, GSK, Amgen, Servier,
2;
N. Franchimont,
Amgen Inc., Biogenidec,
1,
Amgen Inc., Biogenidec,
3;
G. Bianchi,
Amgen, Merck Sharp and Dohme, Novartis, Pfizer, Roche,
8;
R. Chapurlat,
Amgen, Eli Lilly, Ipsen, Servier, Roche, Merck, Novartis,
5;
F. G. Hawkins,
None;
D. L. Kendler,
Amgen,
2,
Amgen,
5,
Amgen,
8;
B. Oliveri,
None;
J. R. Zanchetta,
Amgen, Eli Lilly, MSD, Radius Inc,
2,
Amgen, Eli Lilly, MSD, GSK, Pfizer,
5;
N. Daizadeh,
Amgen Inc.,
1,
Amgen Inc.,
3;
A. Wang,
Amgen Inc.,
3,
Amgen Inc.,
1;
R. B. Wagman,
Amgen Inc.,
1,
Amgen Inc.,
3;
S. Papapoulos,
Amgen Inc., Merck Co., Novartis, Eli Lilly, GSK,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relationship-between-changes-in-bone-mineral-density-and-incidence-of-fracture-with-6-years-of-denosumab-treatment/