Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: SLE is characterized by heterogeneous clinical presentation and periods of flare and remission, with variation in management globally. A goal for SLE management is to achieve and maintain remission or low disease activity to minimize long-term accrual of organ damage. This goal of this study is to assess the frequency, severity, and accrual of damage in patients with SLE as a function of disease activity using LupusNet, the largest existing federated data network in SLE. LupusNet combines and harmonizes data from 5 existing SLE registries, which enables data consistency and enhances understanding of global clinical presentations and outcomes of SLE.
Methods: Data from patients with ≥3 years of follow-up and ≥3 follow-up visits in 3 of 5 LupusNet registries, APLC (Asia Pacific), RELESSER (Europe), and Almenara (South America), were analyzed using a privacy-preserving federated data network approach where only aggregated results were shared. Disease activity was assessed using SLEDAI (recorded at least once a year) and organ damage was measured using SLICC Damage Index (SDI score >0). High disease activity (HDA) was defined as a SLEDAI score >8 at ≥1 visit. The proportion of patients experiencing HDA over the 3-year observation period and the presence of organ damage were reported.
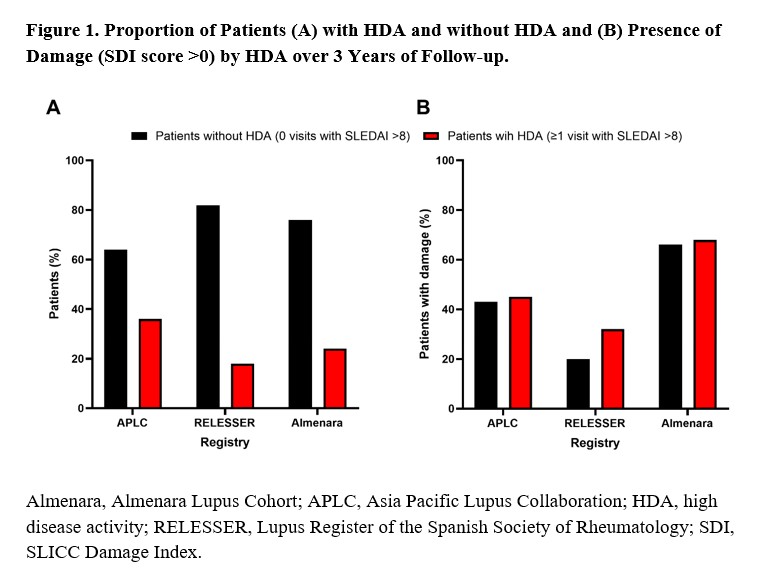
Results: A total of 5019 patients from LupusNet were included. At baseline, 34%, 41%, and 48% of patients had damage in APLC, RELESSER, and Almenara, respectively. At any time over the 3-year follow up, 36% of patients in APLC, 18% in RELESSER, and 24% in Almenara experienced HDA; after 3 years, damage occurred in 44% of patients in APLC, 23% in RELESSER, and 66% in Almenara, and was similar between patients with HDA (32%-68%) and without HDA (20%-66%) across cohorts (Figure 1). However, renal, ocular, and skin damage were more prevalent in patients who experienced HDA compared to those without HDA (Figure 2). Regional variations were evident in the types of organ damage observed, with renal and musculoskeletal damage being consistently prominent across regions. Notably, ocular damage was more prevalent in RELESSER, while gastrointestinal damage was more prevalent in Almenara. The prevalence of most types of organ damage was lower in APLC compared to Almenara and RELESSER. When analyzed by the proportion of visits with HDA, patients who had SLEDAI >8 at more visits exhibited higher rates of proteinuria, haematuria, urinary casts, and pyuria compared to patients without visits with SLEDAI >8. Low complement and high DNA binding were commonly observed irrespective of disease activity (Figure 3).
Conclusion: Despite the well-known link between disease activity and damage accrual, this analysis revealed similar overall rates of damage between patients with and without HDA. However, renal, ocular, and skin damage were more prevalent in patients with HDA. Moreover, regional variations in damage types underscore the complexity of SLE. These findings provide further insights into damage accrual as a function of disease activity in patients with SLE, highlighting the impact of disease activity on certain types of damage and the need for further research to identify factors driving these disparities.
To cite this abstract in AMA style:
Ugarte-Gil M, Gasman S, Zazzetti F, Orillion A, Sheahan A, Blacketer C, van Speybroeck M, Sonmez R, Noss E, Gamboa-Cárdenas R, Pimentel-Quiroz V, Michaud K, Katz P, Kandane-Rathnayake R, Morand E, Louthrenoo W, Hoi A, Chen Y, Cho J, Hamijoyo L, Luo S, Navarra S, Nikpour M, Pego-Reigosa J, Rúa-Figueroa I, Plaza Z, Galindo-Izquierdo M, Martínez Barrio J, Calvo J, Fernández-Nebro A, Menor Almagro R, TOMERO MURIEL E, Narváez J, Karyekar C. Regional Variability in SLE Damage Accumulation by Disease Activity Across the Lupus Federated Data Network (LupusNet) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/regional-variability-in-sle-damage-accumulation-by-disease-activity-across-the-lupus-federated-data-network-lupusnet/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/regional-variability-in-sle-damage-accumulation-by-disease-activity-across-the-lupus-federated-data-network-lupusnet/

.jpg)
.jpg)