Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients (pts) receiving adalimumab for RA or other indications are offered participation in the AbbVie pt support program (PSP). This analysis assessed clinical outcomes across 4 regional areas for pts in the PASSION study according to PSP participation.
Methods: PASSION was a 78-wk postmarketing, global, observational study that included pts with moderate to severe RA receiving adalimumab in routine clinical care. Clinical outcomes were evaluated in PSP users and non-users in North America, Eastern Europe, Western Europe, and Oceania. Outcome measures included proportion of pts with low disease activity (LDA), defined for this analysis as Clinical Disease Activity Index (CDAI) ≤10, Disease Activity Score 28(CRP) (DAS28[CRP]) <3.2, and Simplified Disease Activity Index (SDAI) ≤11; remission, defined for this analysis as CDAI ≤2.8, DAS28-CRP <2.6,and SDAI ≤3.3; and HAQ Disability Index Minimum Clinically Important Difference (HAQ-DI MCID, ≥0.22 decrease from baseline). Differences between PSP users and non-users for demographics and outcome measures were evaluated using a Chi-square test and differences for baseline (BL) disease activity using a 1-way ANOVA.
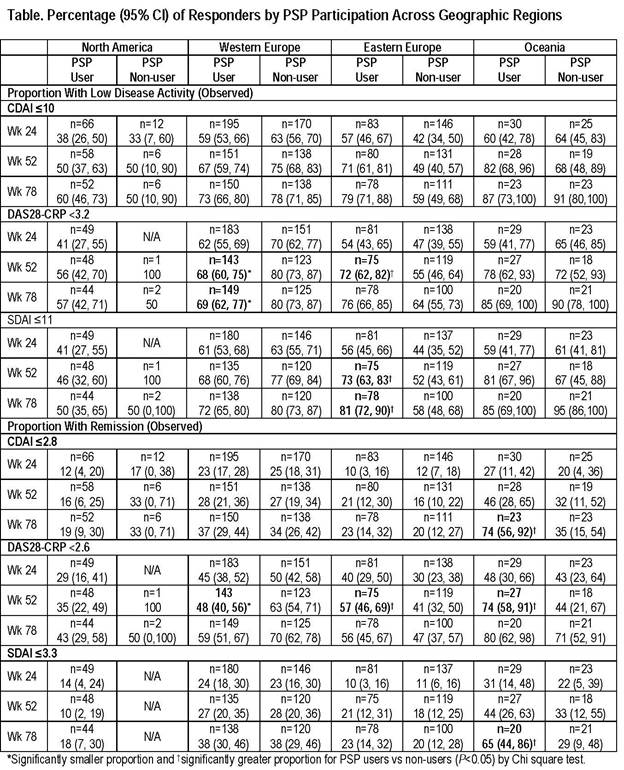
Results: Of 1025 pts receiving adalimumab, 499 were PSP users and 526 were PSP non-users. Most BL characteristics were similar for PSP users and PSP non-users for each region, although prior biologic DMARD use was higher in PSP non-users (18%–29%) vs PSP users (0–16%) across regions (significantly higher for Western Europe and Oceania, P<0.01). BL mean disease activity was similar in PSP users and non-users from North America and Western Europe, but higher in PSP users vs PSP non-users from Eastern Europe (CDAI, DAS28[CRP], SDAI, all P<0.001) and Oceania (CDAI, P<0.05). Responder data analyzed by region for PSP users and non-users suggested that greater proportions of pts achieved LDA and remission with continued adalimumab treatment (Table). Significantly greater proportions of PSP users vs non-users from Eastern Europe and Oceania achieved LDA and/or remission at some time points (P<0.05). The majority of PSP users and PSP non-users achieved HAQ-DI MCID at all assessed timepoints across all 4 regions (53%–87% PSP users; 59%–73% PSP non-users).
Conclusion: With continued adalimumab therapy, greater proportions of both PSP users and PSP non-users achieved LDA and remission. Participation vs non-participation in the PSP was associated with greater achievement of LDA and remission in the Eastern Europe and Oceania populations at some time points.
Acknowledgments:
AbbVie funded the study, contributed to the design, collection, analysis, and interpretation of the data, and in the writing, review, and approval of the abstract. Medical writing support was provided by Catherine DeBrosse, PhD, and Janet Matsuura, PhD, of Complete Publication Solutions, LLC (North Wales, PA, USA) and was funded by AbbVie.
To cite this abstract in AMA style:
van Den Bosch F, Ostor A, Zueger P, Wu M, Lagunes Galindo I, Wassenberg S. Regional Analysis of Impact of Participation in a Patient Support Program on Clinical Outcomes Among Patients with Rheumatoid Arthritis Receiving Adalimumab (Humira) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/regional-analysis-of-impact-of-participation-in-a-patient-support-program-on-clinical-outcomes-among-patients-with-rheumatoid-arthritis-receiving-adalimumab-humira/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/regional-analysis-of-impact-of-participation-in-a-patient-support-program-on-clinical-outcomes-among-patients-with-rheumatoid-arthritis-receiving-adalimumab-humira/
