Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Cystoid Macular Edema (CME) is the leading cause of blindness in non-infectious uveitis. Behçet’s disease (BD) is one of the diseases most frequently associated with CME. Our aims wereto compare the efficacy and safety of Certolizumab Pegol (CZP) and Adalimumab (ADA) in CME due to BD refractory to conventional therapy.
Methods: multicenter study of patients with CME secondary to BD refractory to glucocorticoids (GC) and at least 1 conventional immunosuppressant. All patients had CME (OCT >300µ) at baseline. Efficacy was assessed with the following ocular parameters: macular thickness (µm), visual acuity (BCVA) and GC-sparing effect. The efficacy of CZP vs. ADA was compared between the baseline visit, 1st and 6th month, and 1st and 2nd year. Statistical analysis was performed with IBM SPSS Statistics v.23.
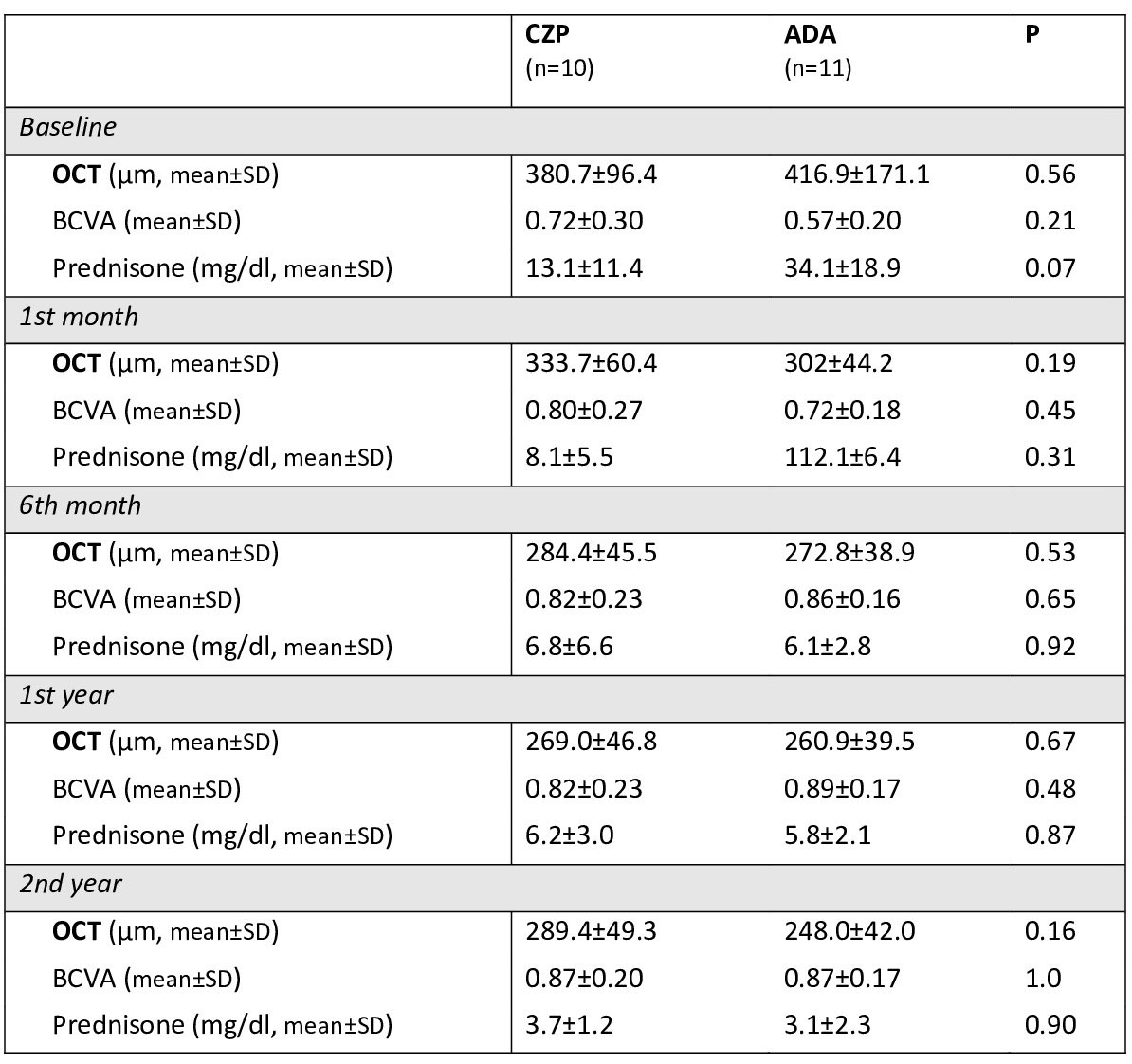
Results: We studied 21 patients/38 affected eyes were studied. 10 patients were treated with CZP (200 mg c/2 weeks) and 11 with ADA (loading dose of 80 mg and subsequently 40 mg c/2 weeks). No statistically significant baseline differences were observed in both groups (CZP vs. ADA) in sex (♂/♀; 3/7 vs 5/6; p=0.65) and mean age (36.1±8.0 vs 42.2±8.6; p=0.10). However, CZP group was more severe with a longer time between EB diagnosis and biologic initiation (91.6±71.4 vs 34.4±21.3 months, p=0.02), and a greater median [IQR] number of previous biologic drugs (2 [0.75-3] vs 0 [0-0]). In CZP group, 8 patients were previously treated with ADA. Combined therapy with conventional DMARDs was used with ADA in 81.8% vs. 18.2% of CZP patients. Regarding the efficacy outcomes analyzed, a rapid and maintained improvement in macular thickness, measured by OCT, was observed after 2 years of follow-up in both groups with no statistically significant differences between them (TABLE). Improvement in visual acuity and a GC-sparing effect was also observed (TABLE). No serious adverse events were observed in either group.
Conclusion: Our study suggests that both CZP and ADA are effective in the treatment of CME due to BD refractory to conventional treatment. CZP was equally effective despite most patients were refractory to ADA.
To cite this abstract in AMA style:
Martín-Varillas J, Sánchez-Bilbao L, Calvo Río V, Adán A, Hernanz Rodríguez I, Cordero Coma M, Díaz Valle D, Fanlo P, de Dios J, Garcia-Aparicio A, Rodriguez Montero S, Jovani Casano V, Moya Alvarado P, Peña Sainz-Pardo e, Hernández J, Blanco R. Refractory Macular Edema Due to Behçet’s Disease. Multicenter Comparative Study of Adalimumab and Certolizumab Pegol [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/refractory-macular-edema-due-to-behcets-disease-multicenter-comparative-study-of-adalimumab-and-certolizumab-pegol/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/refractory-macular-edema-due-to-behcets-disease-multicenter-comparative-study-of-adalimumab-and-certolizumab-pegol/