Session Information
Date: Monday, October 22, 2018
Title: 4M106 ACR Abstract: RA–Treatments III: New Compounds & Biosimilars (1935–1940)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose:
In sarilumab randomized controlled trials (RCTs), dose delay and/or reduction was recommended for management of patients who developed neutropenia Grade (G) 3 (ANC ≥500 to <1000/mm3). This post hoc analysis evaluated the impact of sarilumab dose delay/reduction on efficacy and safety in RCTs and open-label extension (OLE: EXTEND).
Methods:
In RCTs and OLE, patients who experienced G4 or G3 neutropenia and signs of infection permanently discontinued treatment. Patients with G3 neutropenia (no signs of infection) temporarily (or permanently at investigator discretion) discontinued treatment; patients were retested ≤48h after recording decreased ANC and before next scheduled dose, and could resume if ANC ≥1000/mm3. RCTs: patients restarted sarilumab at randomized dose (150 or 200 mg q2w [MOBILITY (NCT01061736)/TARGET (NCT01709578)]; 200 mg q2w [MONARCH (NCT02332590)]). OLE (NCT01146652): patients received sarilumab 200 mg q2w, and restarted sarilumab at 150 mg q2w (per protocol) or 200 mg q2w (investigator discretion); patients requiring dose decrease to 150 mg q2w received that dose for the rest of the study. This analysis included pooled RCT data and OLE data from EXTEND patients enrolling from MOBILITY or TARGET.
Results:
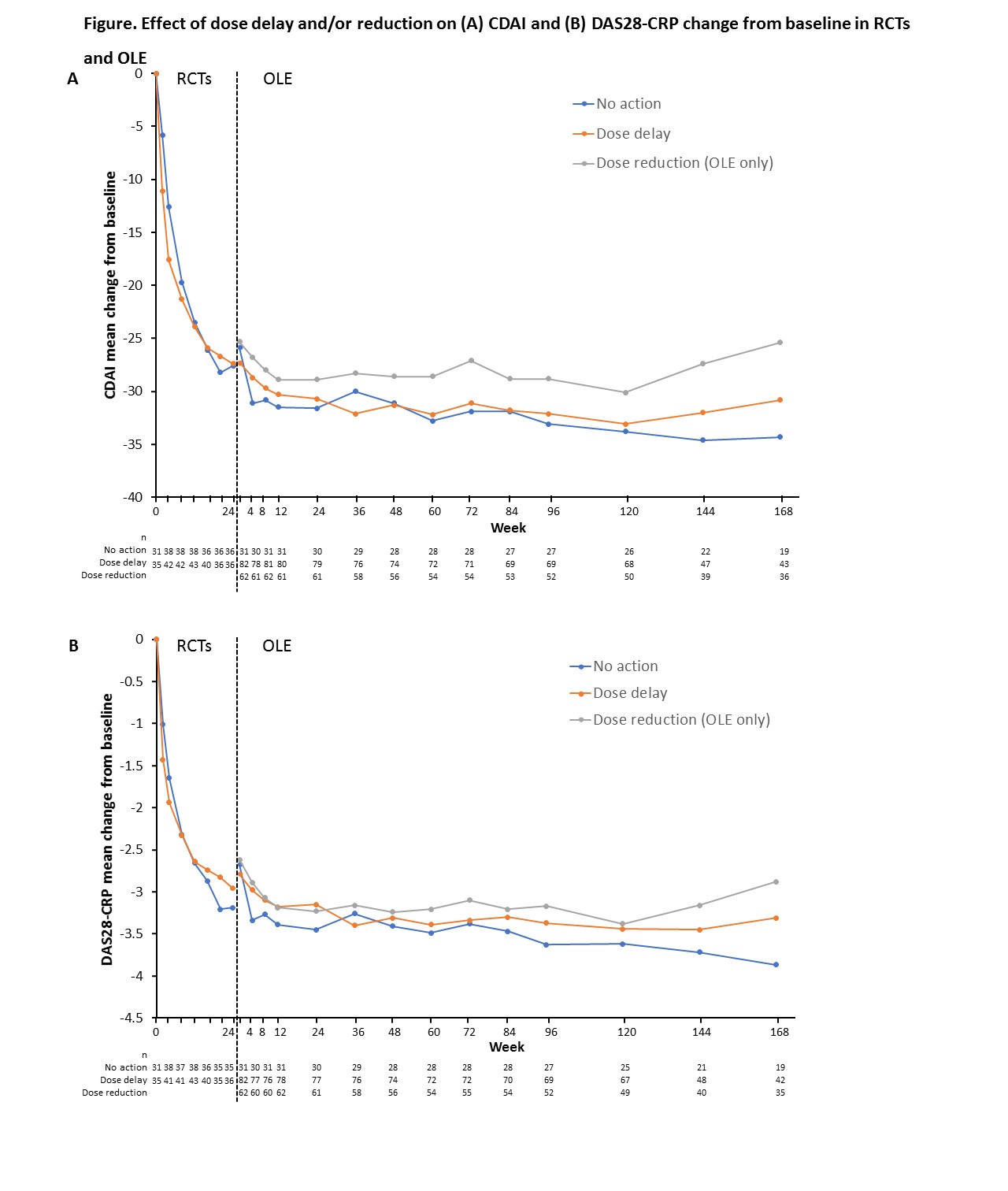
Of the 8–11% of patients experiencing ANC <1000/mm3 at any time (105/1346 [8%] RCT; 147/1353 [11%] OLE), 81/105 [77%] (RCT) and 132/147 [90%] (OLE) were able to continue or reinitiate sarilumab. Of the 81 RCT patients who continued or reinitiated sarilumab, 53% had a dose delay >17 days (i.e. more than one q2w dosing interval) and the remainder continued without interruption. Of 132 OLE patients who continued/reinitiated sarilumab, 82 had dose delay >17 days, 62 had dose reduction, and 31 had no action taken. No meaningful differences in DAS28-CRP or CDAI mean change from baseline over time were observed between patients with ANC <1000/mm3 who reinitiated sarilumab and those without neutropenia who continued without interruption. Among patients with ANC <1000/mm3 who continued treatment, no meaningful differences in CDAI or DAS28-CRP mean change from baseline (Figure) were observed in RCT patients with or without dose delay or in OLE patients with no action/dose delay/dose reduction, although some numerical differences were seen. Patients who were neutropenic at any time did not have an increased risk of infections or serious infections (SIEs) (RCT: infections/SIEs 35.9%/2.4% (non-neutropenia) vs 35.8%/1.2% (neutropenia); OLE: 57.8%/8.5% vs 57.6%/8.3%.
Conclusion:
The majority of patients in RCTs and OLE who temporarily discontinued treatment with sarilumab due to low ANC were able to continue or reinitiate sarilumab with no apparent clinically meaningful impact on long-term efficacy or safety.
Acknowledgements
Study funding and medical writing support (Neil Anderson, Adelphi Communications) provided by Sanofi and Regeneron Pharmaceuticals, Inc.
To cite this abstract in AMA style:
Curtis JR, St. John G, Pannucci M, Lin Y, Maldonado-Cocco JA, Huizinga TWJ, Stanislav M, Emery P. Reductions in Absolute Neutrophil Count (ANC) with Sarilumab Resulting in Dose Delays or Dose Decreases: Effects on Efficacy and Safety [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/reductions-in-absolute-neutrophil-count-anc-with-sarilumab-resulting-in-dose-delays-or-dose-decreases-effects-on-efficacy-and-safety/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reductions-in-absolute-neutrophil-count-anc-with-sarilumab-resulting-in-dose-delays-or-dose-decreases-effects-on-efficacy-and-safety/