Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Compared to the general population, patients (pts) with psoriatic arthritis (PsA) suffer greater amounts of disability and substantially lower employment rates.1 To date there is limited data on the burden of work and household productivity among pts with PsA.2 The results from the RAPID-PsA study indicate significant improvements in work and household productivity with certolizumab pegol (CZP) vs placebo (PBO) up to Week (Wk) 24.3 The purpose of this report is to estimate the economic burden of moderate to severe PsA on productivity and to examine the long-term effect of CZP on workplace and household productivity in RAPID-PsA up to Wk48.
Methods: The ongoing RAPID-PsA trial (NCT01087788) is double-blind and PBO-controlled to Wk24 and dose-blind to Wk48.4 Pts had active PsA and had failed ≥1 DMARD. Pts originally randomized to CZP (200mg Q2W or 400mg Q4W, following 400mg loading dose at Wks 0, 2, 4) continued on their assigned dose in dose-blind phase; PBO pts entering dose-blind phase were re-randomized to CZP loading dose followed by CZP 200mg Q2W or CZP 400mg Q4W. The arthritis-specific Work Productivity Survey (WPS),5 administered Q4W from baseline (BL), assessed the impact of PsA on workplace and household productivity in the randomized set (RS). Disease burden was evaluated at study BL. WPS responses (LOCF imputation) in both CZP groups are summarized descriptively over 48 wks.
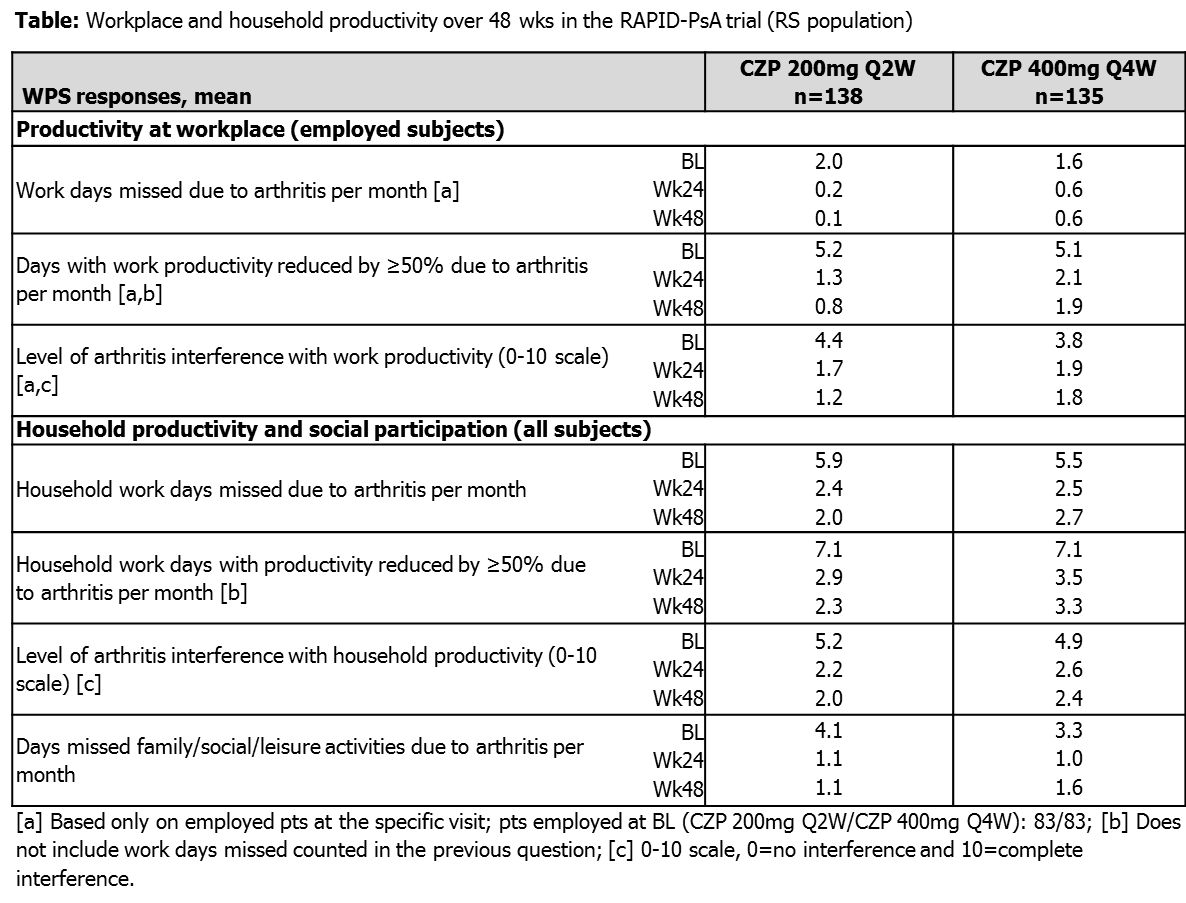
Results: At BL, pts had a mean age of 48 years and 55% were female; 61.6% had psoriasis skin involvement ≥3% body surface area. 59.5% of pts were employed outside the home, 14.0% were work disabled due to PsA and 13.5% were retired at study BL. A high burden of PsA on workplace and household productivity was reported at study BL, with on average >1 wk of paid work affected (mean 6.7 days), ~2 wks of household duties (mean 13.3 days), and mean 3.7 days of social activities affected over previous month. In employed patients in both CZP groups, decreases in absenteeism and presenteeism reported to Wk24 were sustained up to Wk48 (Table). Additionally, the improvements in household productivity and increased participation in social activities reported in both CZP groups over 24 wks were maintained up to Wk48 (Table).
Conclusion: PsA is associated with a high burden of disease on workplace and household productivity that could lead to large financial burden for pts and society. This analysis indicates that CZP improved workplace productivity in patients with PsA by reducing absenteeism and presenteeism and improved household productivity and increased participation in social and daily activities. The benefits of CZP were maintained up to Wk48.
References: 1. Mau W. J Rheumatol 2005;32:721-728; 2. Tillet W. Rheumatology 2012;51:275-283; 3. Kavanaugh A. Value in Health 2013;16:A228; 4. Mease P. Arthritis Rheum 2012;64(10):1107; 5. Osterhaus J. Arth Res Ther 2009;11(3):R73
Disclosure:
A. Kavanaugh,
Abbott, Amgen, Bristol-Myers Squibb, Pfizer, Roche, Janssen, UCB Pharma,
2;
D. D. Gladman,
Abbott, Bristol-Myers Squibb, Celgene, Johnson & Johnson, MSD, Novartis, Pfizer, UCB Pharma,
2,
Abbott, Bristol-Myers Squibb, Celgene, Johnson & Johnson, MSD, Novartis, Pfizer, UCB Pharma,
5;
D. M. van der Heijde,
AbbVie, Amgen, AstraZeneca, Augurex, Bristil-Myers Squibb, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Vertex,
5,
Imaging Rheumatology bv.,
9;
O. Purcaru,
UCB Pharma,
3;
P. J. Mease,
AbbVie, Amgen, BiogenIdec, Bristol-Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Eli-Lilly, Merck, Novartis, Pfizer, UCB Pharma, Vertex,
2,
AbbVie, Amgen, BiogenIdec, Bristol-Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Eli-Lilly, Merck, Novartis, Pfizer, UCB Pharma, Vertex,
5,
AbbVie, Amgen, BiogenIdec, Bristol-Myers Squibb, Crescendo, Genentech, Janssen, Eli-Lilly, Pfizer, UCB Pharma,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduction-of-disease-burden-on-workplace-and-household-productivity-in-psoriatic-arthritis-over-48-weeks-of-treatment-with-certolizumab-pegol/