Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Glucocorticoids (GC) play an important role in rapid systemic lupus erythematosus (SLE) symptom relief. However, chronic GC use increases organ damage risk; and treatment recommendations are to minimize/discontinue GC use when clinically possible.1 Belimumab (BEL) is a disease-modifying SLE treatment that demonstrated a consistent efficacy profile in 4 Phase 3 trials.2–5 This study analyzed pooled SLE data to assess whether GC reduction occurred more rapidly in patients (pts) randomized to BEL versus placebo (PBO).
Methods: Belimumab Summary of Lupus Efficacy (Be-SLE) analyzed data from 5 double-blind, PBO-controlled BEL studies in SLE: BLISS-76, BLISS-52, BLISS-NEA, BLISS-SC, and EMBRACE. The analysis compared BEL (10 mg/kg intravenous [IV] or 200 mg subcutaneous [SC]) versus PBO (IV and SC) pooled data, collected every 4 weeks from baseline to Week 52. Although GC tapering was recommended for pts responding well to treatment, GC adjustments were at the investigator’s discretion. GC use was converted to prednisone-equivalent dose (mg/day). A ≥25% GC reduction from baseline to ≤7.5 mg/day at Week 52 was a key GC endpoint in all trials. Odds ratios (OR) and 95% confidence intervals (CI) for GC dose changes were calculated using a logistic regression model with covariates of treatment, study, baseline GC dose, and (SELENA-SLEDAI score (≤9 versus ≥10).
Results: The analysis included 1869 and 1217 pts receiving BEL and PBO, respectively. Most pts were female (94.4%), with a median age of 36.0 years (range 18–77 years); baseline demographics and disease characteristics were balanced between groups. Most pts (BEL 88.2% [n=1648]; PBO 88.7% [n=1079]) received GC at baseline; mean baseline GC dose for BEL and PBO was 12.3 and 12.2 mg/day, respectively. A ≥25% GC reduction from baseline to ≤7.5 mg/day during Week 40 through Week 52 was achieved by 16.9% (n=202) of BEL recipients versus 11.9% (n=91) of PBO patients (OR: 1.52 [95% CI: 1.16–1.99], p=0.0024).
At Week 52, 33.9% of pts receiving BEL had a GC decrease from baseline, versus 27.4% with PBO (OR: 1.41 [95% CI: 1.18–1.70], p=0.0002; Figure). Conversely, 11.1% and 16.2% of BEL and PBO pts had a GC increase, respectively (OR: 0.65 [95% CI: 0.52–0.81], p=0.0001; Figure). Statistically significant differences in GC use between BEL and PBO were observed as early as Week 12 for “any increase in GCs,” and by Week 24 for “any decrease in GC” (Figure). Mean (standard deviation) cumulative GC dose over 1 year was 4495 mg (4018.8) with BEL and 5096 mg (5641.6) with PBO (p=0.024 by analysis of covariance).
Conclusion: BEL was associated with significantly greater reductions in GC dose from baseline, and a lower cumulative GC dose over 1 year versus PBO. GC reductions with BEL occurred early in the 52-week treatment period despite no forced taper in the original studies. These data further support the role of BEL as a GC-sparing treatment in SLE management.
Funding: GSK
References:
1Smolen JS, et al. Ann Rheum Dis 2020;79(6):685–99
2Navarra SV, et al. Lancet 2011;377(9767):721–31
3Furie RA, et al. Arthritis Rheum 2011;63(12):3918–30
4Zhang F, et al. Ann Rheum Dis 2018;77(3):355–63
5Stohl W, et al. Arthritis Rheumatol 2017;69(5):1016–27
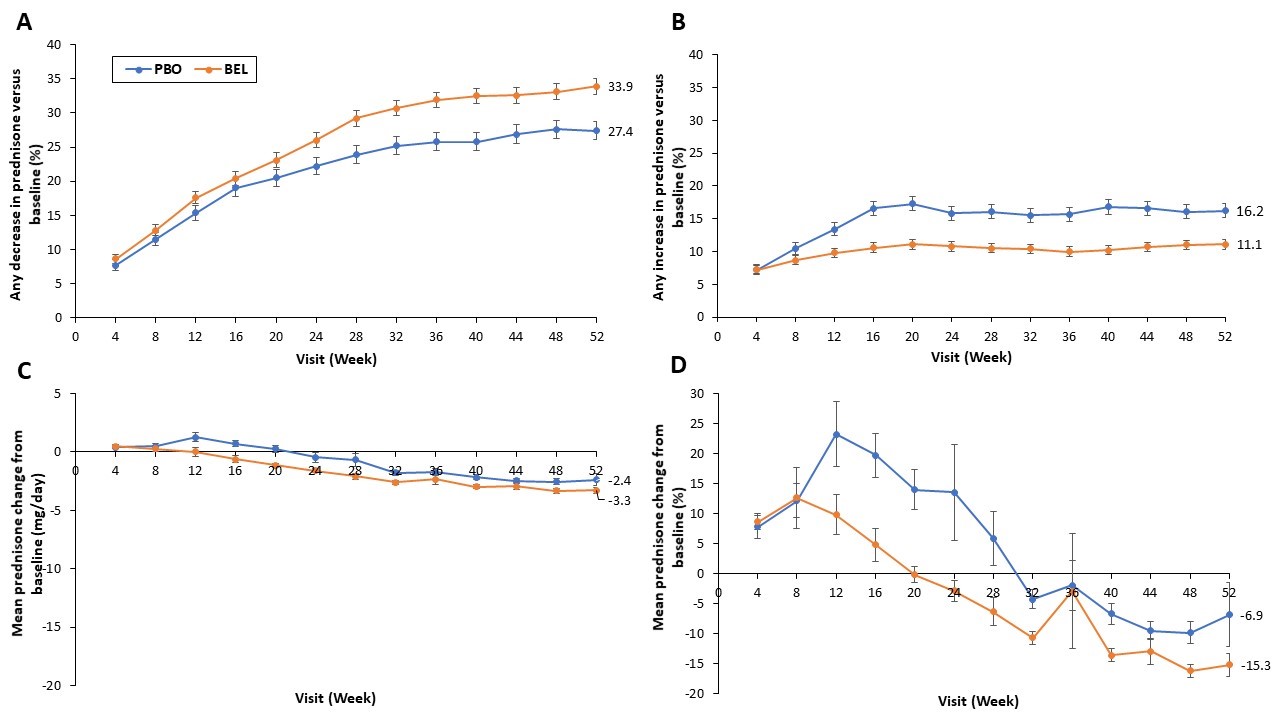 Figure. Percentage of pts with a decrease in GC dose compared with baselinea at each visit (A), percentage of pts with an increase in GC dose compared with baselineb at each visit (B), mean change from baselineb in GC dose (mg/day) at each visit (C); mean percentage change from baselinea in GC dose at each visit (D) Error bars represent the standard error. Numerical values shown represent data at Week 52. aAnalysis of pts receiving GCs at baseline bAnalysis of all pts, including those receiving GCs at baseline
Figure. Percentage of pts with a decrease in GC dose compared with baselinea at each visit (A), percentage of pts with an increase in GC dose compared with baselineb at each visit (B), mean change from baselineb in GC dose (mg/day) at each visit (C); mean percentage change from baselinea in GC dose at each visit (D) Error bars represent the standard error. Numerical values shown represent data at Week 52. aAnalysis of pts receiving GCs at baseline bAnalysis of all pts, including those receiving GCs at baseline
To cite this abstract in AMA style:
Costenbader K, Abe Y, Arnaud L, Bertsias G, Fox N, Gibb M, Hammer A, Meara A, Quasny H, Roth D, Gonzalez-Rivera T. Reduction in Glucocorticoid Use in Patients with Systemic Lupus Erythematosus Treated with Belimumab: A Large Pooled Analysis of 5 Placebo-Controlled Studies [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/reduction-in-glucocorticoid-use-in-patients-with-systemic-lupus-erythematosus-treated-with-belimumab-a-large-pooled-analysis-of-5-placebo-controlled-studies/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduction-in-glucocorticoid-use-in-patients-with-systemic-lupus-erythematosus-treated-with-belimumab-a-large-pooled-analysis-of-5-placebo-controlled-studies/
