Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Nonvertebral fracture risk is significantly influenced by cortical thickness, area, mass, and porosity because all of these contribute to bone strength. In particular, increased cortical porosity, a marker of structural decay, is associated with an exponential worsening in bone fragility. Cortical porosity is the result of unbalanced and accelerated intracortical remodeling upon Haversian canal surfaces which enlarge, coalesce, and fragment the cortex. Reducing remodeling will limit worsening of porosity but for individuals already at increased risk for fracture, reducing porosity is a preferred outcome. Using multi detector computer tomography (MDCT) hip images from the FREEDOM study, we previously reported that hip cortical mass and thickness improved over 3 years of denosumab (DMAb) administration and postulated that this could be explained by infilling of porosity in the inner cortical region adjacent to the medullary canal. Here, we used a subset of these images to evaluate changes in hip porosity.
Methods: FREEDOM was a 3-year, randomized, double-blind trial that enrolled postmenopausal women with a lumbar spine or total hip T-score <–2.5 but not <–4.0 at both sites. Women were randomly assigned to placebo (Pbo) or 60 mg DMAb every 6 months. Percentage porosity in both the compact and the trabecularized (outer and inner transitional zones) cortical volumes of the subtrochanter region were measured using StrAx1.0 software (Zebaze et al., Bone 2013) from MDCT hip images obtained at baseline and year 3 (Pbo, n=22; DMAb, n=28).
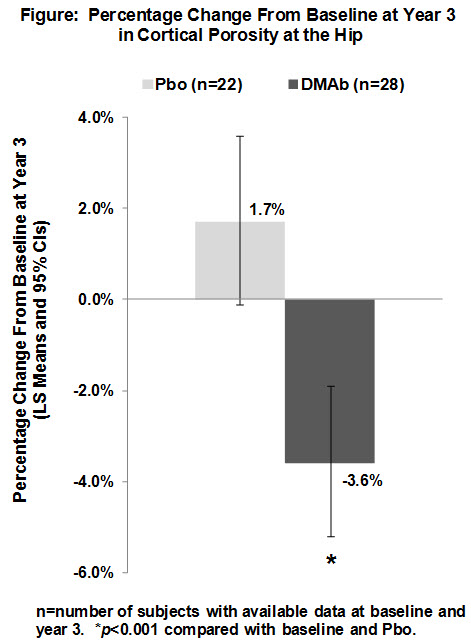
Results: Cortical porosity was larger as a function of proximity to the medullary canal; the percentage volume occupied by porosity at baseline was 72% in the inner transitional zone adjacent to the medullary compartment, 37% in the outer transitional zone, and 29% in the compact-appearing cortex. Cortical porosity correlated positively with serum CTX (p=0.017) and negatively with hip strength estimated using finite element analysis (p=0.027). DMAb reduced porosity compared with baseline and Pbo at year 3 across the entire cortex (Figure) and in each cortical sub-compartment, reaching treatment effect (DMAb–Pbo) improvements of –1.8% (inner transitional zone), –5.6% (outer transitional zone), and –7.9% (compact-appearing cortex) (all p<0.001).
Conclusion: This is the first report of in vivo hip porosity changes in response to a pharmacological therapy. Since reductions in cortical porosity equate to increased mineralized bone matrix mass and both are relevant to strength, these improvements are expected to contribute to the observed reductions in nonvertebral fractures associated with DMAb administration.
Disclosure:
R. M. Zebaze,
None;
C. Libanati,
Amgen,
1,
Amgen,
3;
M. R. McClung,
Amgen, Lilly, Merck,
5,
Amgen, Merck, Novartis, Warner-Chilcott,
7,
Amgen, Merck,
2;
J. R. Zanchetta,
Amgen, Eli Lilly, MSD, GSK,
5,
Amgen, MSD, Radius Inc.,
2;
D. L. Kendler,
Amgen, Eli Lilly, Merck, Novartis, Pfizer,
5,
Amgen, Eli Lilly, Novartis, Pfizer, Warner Chilcott,
7,
Amgen, Eli Lilly, Novartis, Pfizer, GSK, J and J,
2;
A. Høiseth,
None;
A. Wang,
Amgen,
1,
Amgen,
3;
A. Ghasem-Zadeh,
University of Melbourne,
3,
StrAx Corp – one of the inventors of StrAx 1.0 (analysis software),
4;
E. Seeman,
Amgen, Sanofi Aventis, MSD, Eli Lilly, Novartis, Warner Chilcott,
5,
Amgen, MSD,
7,
Amgen, MSD,
2,
Director StraxCorp,
4.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduced-hip-cortical-porosity-upon-denosumab-treatment-a-likely-mechanism-contributing-to-the-reduction-of-hip-fracture-risk-in-women-with-osteoporosis/