Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Regulatory guidance on endpoint measures for disease activity in cutaneous lupus erythematosus (CLE) patients is essential to improve therapies. CLE profoundly impacts quality of life (QoL), and improvement in disease activity by the Cutaneous Lupus Disease Area and Severity Index (CLASI) is associated with QoL improvement.1,2 Thus, QoL should be a key consideration in trial eligibility and endpoint measure determination.
Current trial inclusion criteria often limits eligibility to those with moderate to severe disease activity (CLASI-A ≥10), excluding patients with mild disease activity (CLASI-A ≤9) due to lack of validated efficacy measures.3 Our group recently found the established efficacy measure of 50% CLASI improvement (CLASI50) is predictive of meaningful QoL change in patients with a baseline score ≥8, suggesting that patients with milder disease can benefit from trial inclusion.4 Thus, we sought to further assess the QoL in patients with CLASI scores of 8-9 to determine the suitability including those patients in clinical trials.
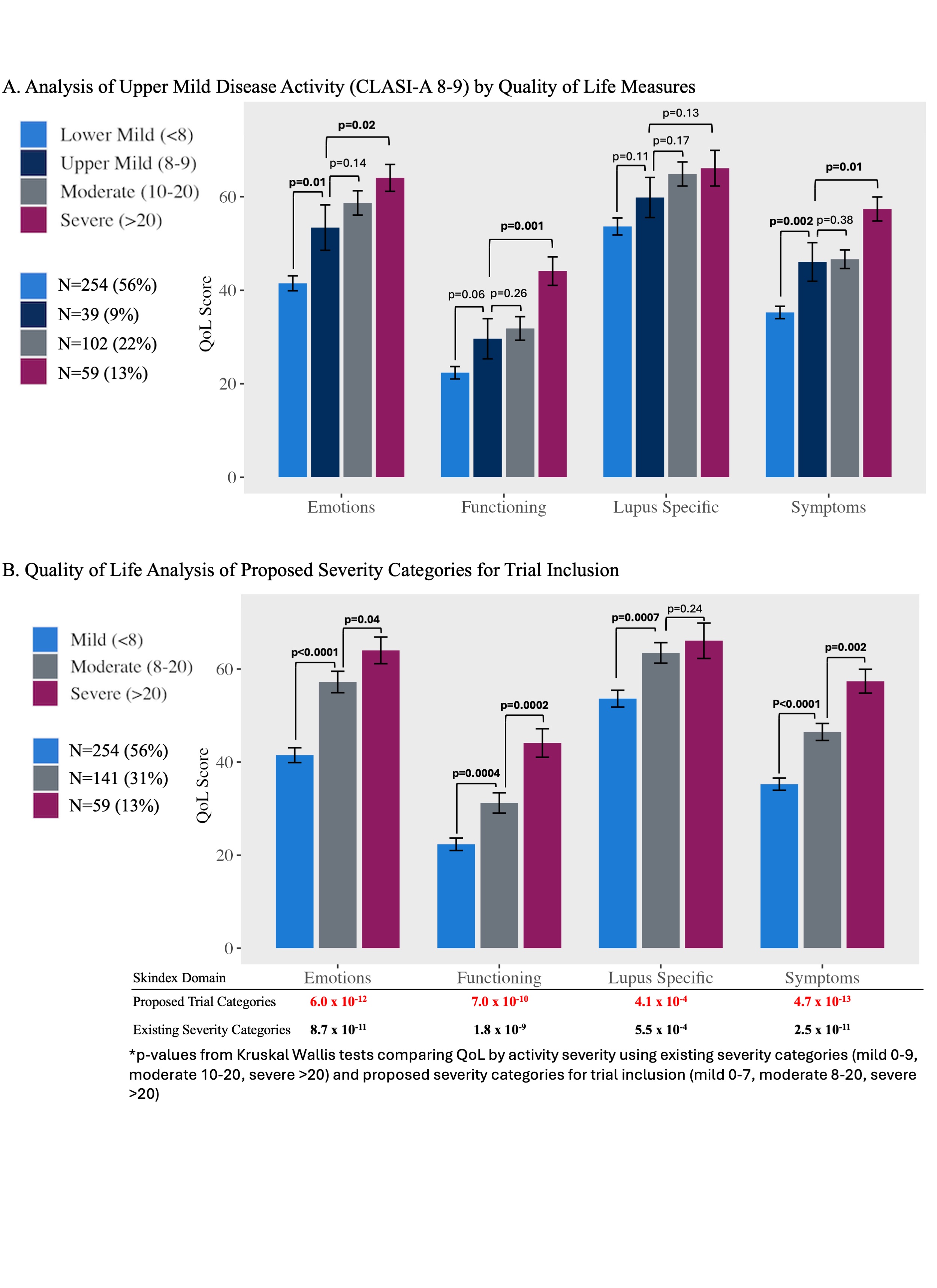
Methods: Patients from the University of Pennsylvania Longitudinal CLE Database study with at least one complete visit were included, with the first complete visit used for analysis (n=454). Activity severity categories for analysis were defined as lower-mild (0-7: n=254, 56%), upper-mild (8-9: n=39, 9%), moderate (10-20: n=102, 22%) and severe ( >20: n=59, 13%) using CLASI-A scores.3 QoL was assessed using the Skindex 29+3. Kruskal Wallis and Dunn’s multiple comparison tests were used to compare Skindex domain scores between disease severity categories.
Results: Results showed that Skindex scores of upper-mild patients differed significantly from lower-mild patients with respect to emotions (mean [SEM]: 54.5 [5.0] vs 41.2 [1.6], p=0.01), and symptoms (46.6 [4.3] vs. 35.1 [1.3], p=0.002), but did not differ significantly from moderate scores in any domain. This suggests that QoL among those with CLASI scores 8-9 is akin to those with moderate disease (Figure 1A). This is further supported by increased significance by Kruskal Wallace testing using the new proposed categories for trial inclusion (Figure 1B) compared to the existing categories of activity severity, indicating increased precision.
Importantly, lowering CLASI eligibility to include CLASI 8-9 also has potential to enable more diverse trial participation. Compared to entry criteria that require a minimum CLASI score of 10, lowering the threshold to ≥8 would increase eligibility of Black patients by 30% (n=56 vs. 43), White patients by 20% (n=130 vs. 108), and other racial groups by 40% (n=14 vs. 10) within our cohort. This is particularly important given the under-representation of minoritized populations in CLE clinical trials and the critical need to ensure drug safety and efficacy are adequately tested across diverse demographics.5
Conclusion: In summary, our findings suggest that QoL in patients with a CLASI score of 8-9 is more aligned with moderate disease activity than with lower-mild activity. This, along with the finding that CLASI50 is predictive of meaningful change in those with a baseline CLASI score ≥8, supports the inclusion of patients with milder activity in clinical trials.
To cite this abstract in AMA style:
Faden D, Xie l, Stone C, Lopes Almeida Gomes L, Eldaboush A, Ricco C, Feng R, Werth V. Redefining Clinical Trial Inclusion Criteria Using Quality of Life in Cutaneous Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/redefining-clinical-trial-inclusion-criteria-using-quality-of-life-in-cutaneous-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/redefining-clinical-trial-inclusion-criteria-using-quality-of-life-in-cutaneous-lupus-erythematosus/