Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In North America, biosimilars were approved only relatively recently, and real-world data are few. We described users of infliximab in the US, comparing patient tolerability of biosimilar and originator, in terms of missed doses and discontinuation.
Methods: We used Marketscan® data (Jan-Dec. 2017) to identify adult (age >18) new users of infliximab biosimilar and originator, and switchers from originator to biosimilar. Characteristics (age, sex, comorbidities, medication use) were described. In new users, we assessed missed doses in both the induction (among subjects with >2 months follow-up) and maintenance phases. ‘Missed dose’ was defined as any gap between infusions beyond recommended intervals (0, 2, and 6 weeks for induction and Q8 weekly for maintenance). Discontinuation (≥ 90-day gap between infusions without restarting therapy) in the maintenance phase was also assessed. We used Cox regression to compare both times to first missed dose and complete discontinuation. All models were adjusted for age, sex, prior use of DMARDs, biologics, and systemic glucocorticoids, comorbidities (Charlson comorbidity index) and underlying disease indication (rheumatoid arthritis, ankylosing spondylitis, psoriasis/psoriatic arthritis, Crohn’s disease, and ulcerative colitis). ).
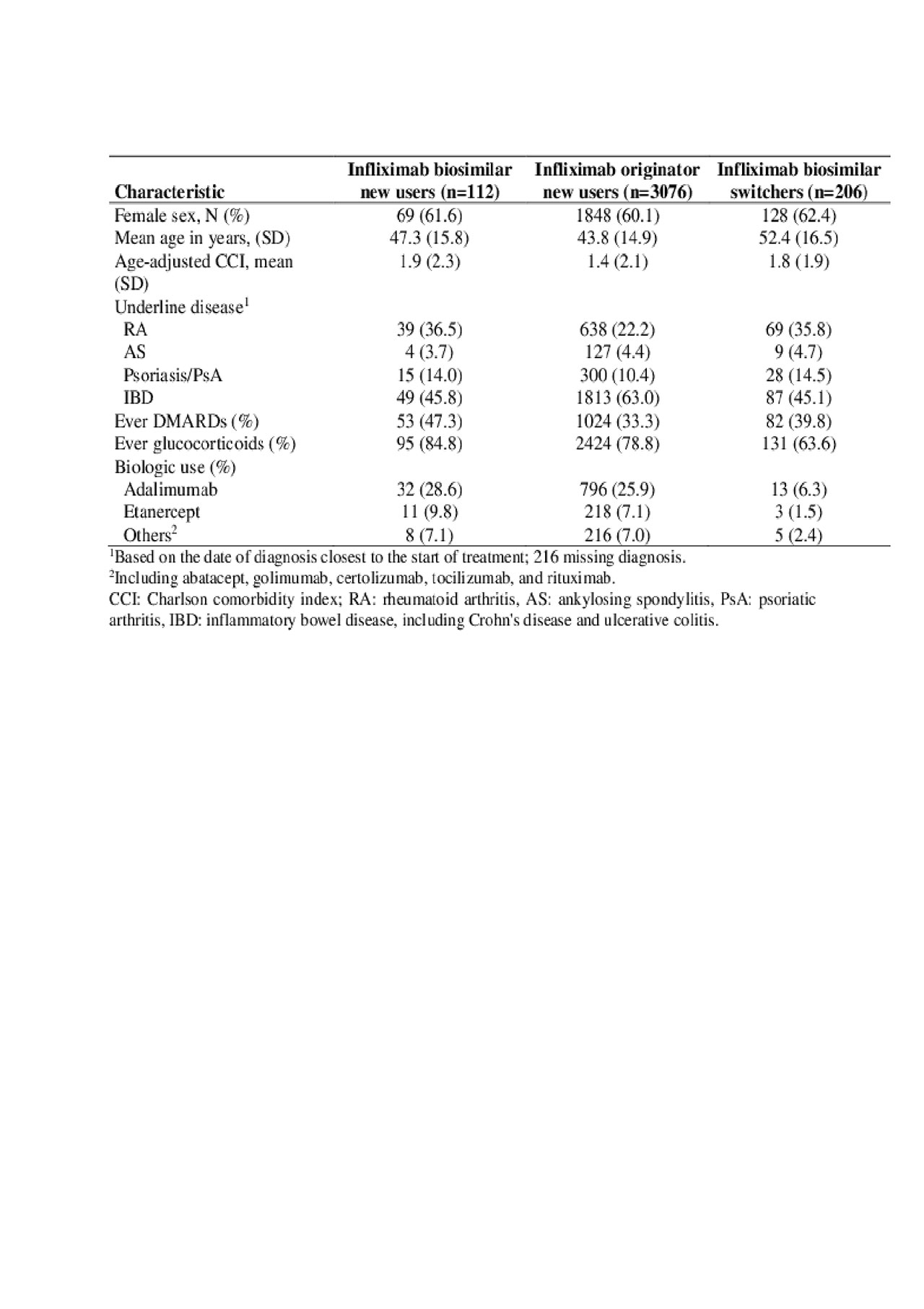
Results: We identified 318 users of infliximab biosimilar, including 206 switchers from the originator infliximab (Table 1). Among the 92 new users of infliximab biosimilar with >2 months follow-up, the frequency of >1 missed dose during induction was 22%, similar to 25% in new users of the originator. For patients completing the induction phase, the adjusted hazard ratio (HR) showed a nonsignificant trend for a longer time to first missing dose in maintenance (adjusted HR 0.33, 95% CI 0.08-1.30, Table 2). We were unable to determine if complete discontinuation differed between the two groups (HR: 0.82; 95% CI: 0.11-6.02).
Conclusion: We documented low use of infliximab use in these US data during 2017; most infliximab biosimilar initiators are switchers from the originator. For previously infliximab-naïve patients, the frequency of >1 missed dose during infliximab induction phase was similar in the originator and the biosimilar new users. As the frequency of biosimilar use grows, additional analyses with more follow-up time may help determine if there are differences in persistence between biosimilars and their reference therapy.
To cite this abstract in AMA style:
Moura C, Curtis J, Choquette D, Boire G, Bykerk V, Thorne C, Maksymowych W, Lakatos P, Svenson L, Targownik L, Afif W, Bernatsky S. Recent Use, Missed Doses and Discontinuation of Infliximab in a Population-Based Cohort: Comparisons of Biosimilar and Originator Exposures [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/recent-use-missed-doses-and-discontinuation-of-infliximab-in-a-population-based-cohort-comparisons-of-biosimilar-and-originator-exposures/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/recent-use-missed-doses-and-discontinuation-of-infliximab-in-a-population-based-cohort-comparisons-of-biosimilar-and-originator-exposures/