Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Provider adherence to a Treat to Target (TTT) strategy for management of rheumatoid arthritis (RA) requires setting a disease target, regular monitoring of disease activity, shared decision-making, and appropriate adjustment of therapy based on target and disease activity score. We conducted a quality improvement trial to test whether a Learning Collaborative would improve TTT implementation. This analysis examines documented reasons for non-adherence to TTT by providers in the intervention arm.
Methods: Medical record review conducted by trained study staff assessed TTT implementation based on 4 components: designation of target (remission or low disease activity), disease activity measure (DAM), shared-decision making (when applicable to set target and/or change treatment), and change in treatment based on target and DAM. Within the Learning Collaborative, providers were encouraged to reports reasons for TTT non-adherence when treatment was not changed despite disease activity not achieving target. Provider non-adherence to TTT was defined as no treatment intensification when warranted by disease activity not at pre-determined target. Reasons for non-adherence were categorized as: patient-based preferences (i.e. financial barriers, fear of medication, and reluctance to change), drug side effects, elevated DAM unrelated to RA (i.e. pain from comorbid conditions or irreversible joint damage increases score), no active disease as deemed by provider examination, treatment contraindicated (i.e. pregnancy, renal disease, chemotherapy), medication time-lag (i.e. delayed treatment response), other, and no reason specified. Multiple reasons were possible for each visit. Patient DAM scores were classified based on CDAI or RAPID3.
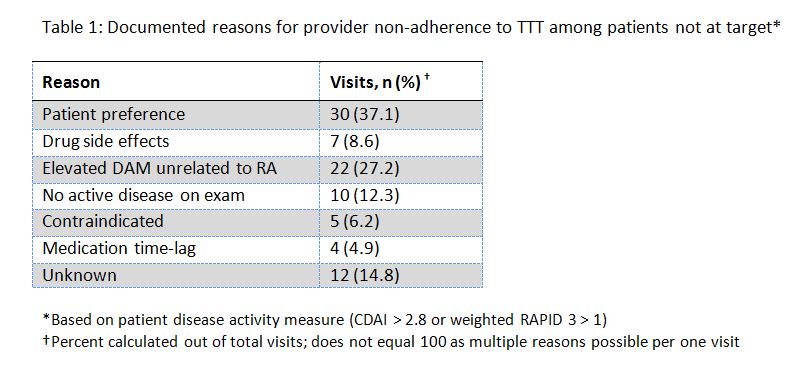
Results: 81 visits with provider non-adherence to the TTT strategy were observed in 74 patients, distributed throughout the nine month study period. A total of 90 reasons for non-adherence were noted. Patient preference was the most frequently observed reason for provider non-adherence to TTT, cited in 30 (37.1%) visits (see Table 1). Of the 81 visits, 22 (27.2%) visits noted that the DAM was elevated but not due to RA, and 12 (14.8%) had an unspecified reason for non-adherence.
Conclusion: Patient preference was the leading documented reason for provider non-adherence to a TTT strategy in RA, highlighting the importance of patient involvement in the treatment decision-making process. Patients play a critical role within the TTT paradigm.
To cite this abstract in AMA style:
Zak A, Corrigan C, Fraenkel L, Harrold LR, Katz JN, Lee S, Pincus T, Smolen JS, Solomon DH. Reasons for Provider Non-Adherence to a Treat to Target Strategy in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/reasons-for-provider-non-adherence-to-a-treat-to-target-strategy-in-rheumatoid-arthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reasons-for-provider-non-adherence-to-a-treat-to-target-strategy-in-rheumatoid-arthritis/