Session Information
Date: Tuesday, November 12, 2019
Title: Pediatric Rheumatology – ePoster III: Systemic JIA, Fever, & Vasculitis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic juvenile idiopathic arthritis (SJIA) is a rare autoinflammatory disease characterized by fever and arthritis, often accompanied by rash. Canakinumab (CAN) was approved in the United States (US) in 2013 for SJIA treatment. However, prescribing patterns among physicians who have initiated CAN in real world settings are not well understood. This study aimed to assess the physician reason(s) for initiating CAN and characterize the clinical and treatment profiles of SJIA patients (pts) in US clinical practice.
Methods: Online medical charts were reviewed retrospectively to collect data from US rheumatologists/ dermatologists/ allergists / immunologists on pts (pediatrics [Ped; < 18 yrs] and adults [Adt; ≥18 yrs]) diagnosed with SJIA who were initiated on CAN therapy by the responding physician between 2016 and 2018. Online case report forms were used to collect information on pt demographics, disease characteristics at CAN initiation, pre-CAN treatment history, and CAN prescribing patterns. Reasons for discontinuation of previous treatment and initiation of CAN were also collected.
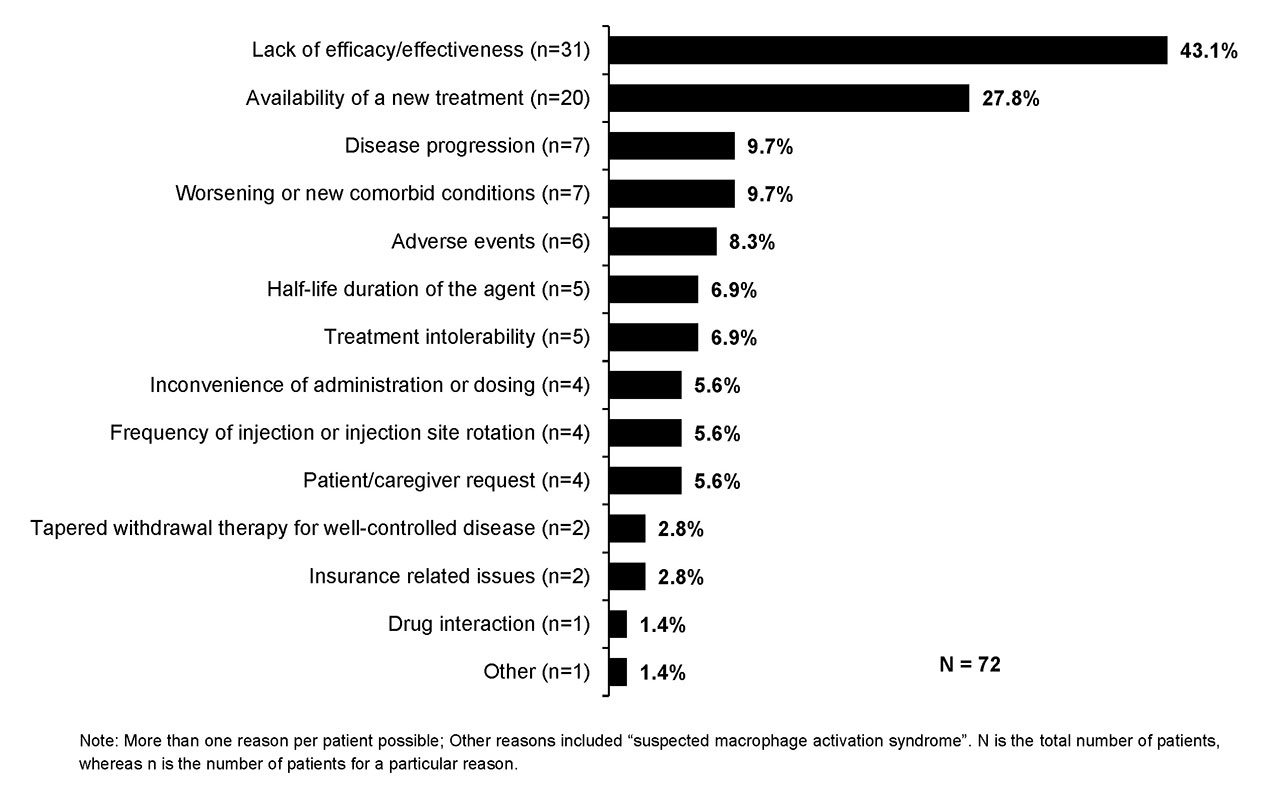
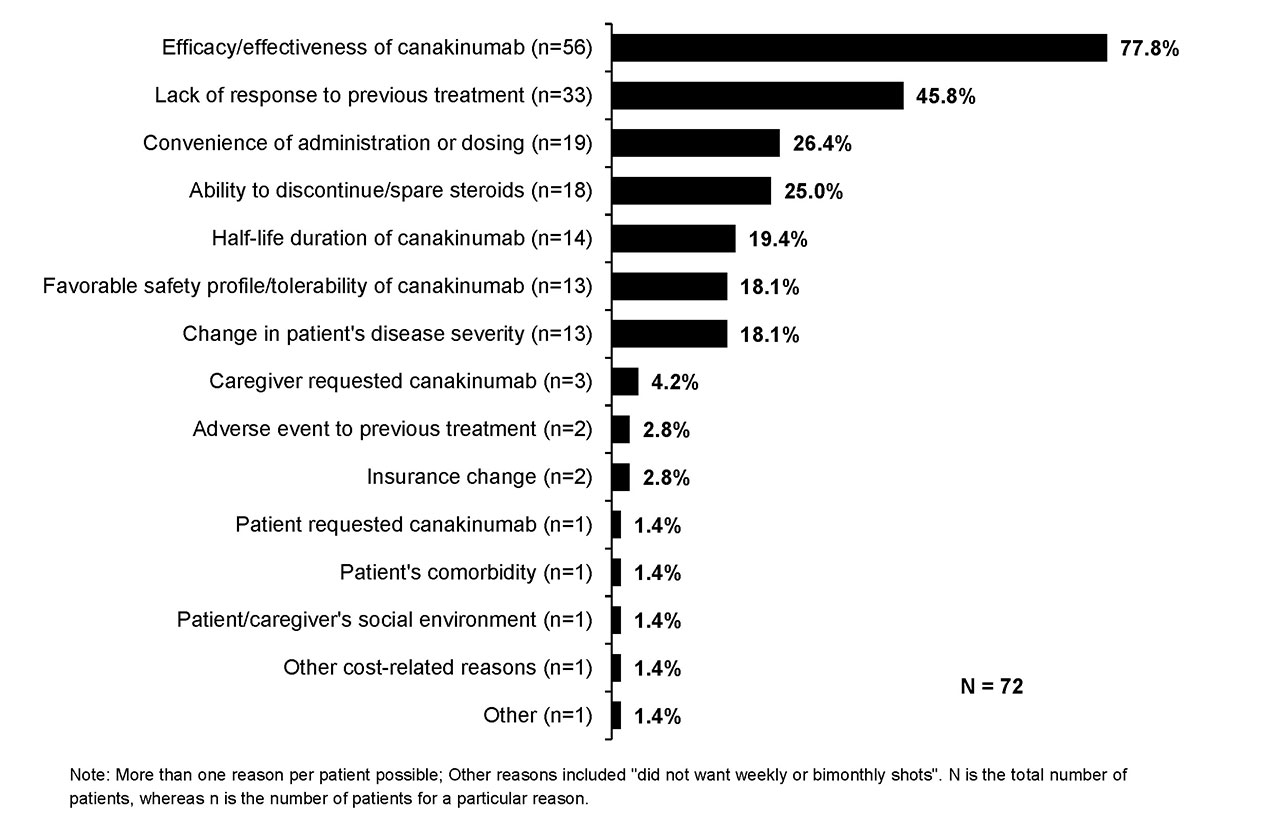
Results: Medical charts were reviewed by 43 physicians who had specialty in rheumatology (22; Adt 82%, Ped 18%), dermatology (12; Adt 75%, Ped 25%), immunology (5; Adt 40%, Ped 60%), and allergy (4; Adt 100%). Of the 72 pts, 57% were female and 61% were Ped. The mean age at CAN initiation was 19.4 yrs (Adt, 33.5 yrs; Ped, 10.4 yrs). At CAN initiation, 71% of pts were with and 29% were without active systemic features. Median age at SJIA diagnosis was 11 yrs (IQR: 6.5-16). The key methods of diagnosis were assessment of clinical symptoms and complications (92%), exclusion/rule-out diagnosis (e.g., infection, neoplasms; 75%), and age of onset (67%). The main diagnoses which were ruled out included fever of unknown origin (76%), other JIA subtype (43%), and other PFS (39%). The severity of SJIA was mild (11%), moderate (75%), or severe (14%). The most common symptoms at CAN initiation were fever (72%), fatigue/malaise (58%), skin rashes (47%), and arthritis (46%). A large part of pts at CAN initiation had 1-4 joints with active inflammation (69%) and 1-4 joints with limited range of motion (63%). Nearly all pts (90%) received other long-term treatments in the last line of therapy prior to CAN, including biologics (e.g., 28% etanercept, 19% anakinra, 17% adalimumab, and 11% tocilizumab), methotrexate (9%), and NSAIDs (8%). The main reasons for discontinuation of treatment prior to CAN initiation were lack of efficacy/effectiveness (43%) and availability of a new treatment (28%; Fig. 1). Decision to initiate CAN was decided most often by both physician and pt/caregiver (72%), followed by physician only (26%), and then by the pt/caregiver only (1%). The prime reasons for CAN initiation included efficacy/effectiveness (78%), lack of response to previous treatment (46%) and convenience of administration or dosing (26%; Fig. 2).
Conclusion: Findings from this study provide insight into physicians’ characteristics in clinical practice as well as the reasons for CAN initiation in SJIA pts. The efficacy/effectiveness of CAN, lack of response to previous treatment and convenience of CAN administration or dosing were the most common reasons.
To cite this abstract in AMA style:
Hur P, Ionescu-Ittu R, Manceur A, Lomax K, Cammarota J, Xie J, Sanghera N, Grom A. Reasons for Initiation of Canakinumab of Patients with Systemic Juvenile Idiopathic Arthritis: A Retrospective Medical Chart Review from the United States [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/reasons-for-initiation-of-canakinumab-of-patients-with-systemic-juvenile-idiopathic-arthritis-a-retrospective-medical-chart-review-from-the-united-states/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reasons-for-initiation-of-canakinumab-of-patients-with-systemic-juvenile-idiopathic-arthritis-a-retrospective-medical-chart-review-from-the-united-states/