Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Rheumatoid arthritis (RA) patients who failed a first biologic agent due to any reasons have the option of switching to a second one along with the strategy of biologic agent treatment. Patients go over switching to the next one at failing their biologic agent. On the other hand, there are some patients who discontinue any biologic agent treatment due to various reasons such as tolerability concern, complications, economic issue, remission and so on. The impact of this concern has been less studied.
The objective of this study was to investigate the reasons and the risk factors for discontinuation any biologic agent in RA patients.
Methods
In total patients (n=2179) who underwent biologic agent treatment between 2003 and 2011 at Nagoya University Hospital and 12 other institutes (Tsurumai Biologics Communication Study Group), 1966 patients who were confirmed continuation or discontinuation of biologic agent treatment were enrolled. We analyzed the retention rate of biologic agent treatment and the reasons for discontinuation. To identify the risks for discontinuation, baseline demographics were compared between the continuing group and the disccontinuing group using cox hazard regression analysis.
Results
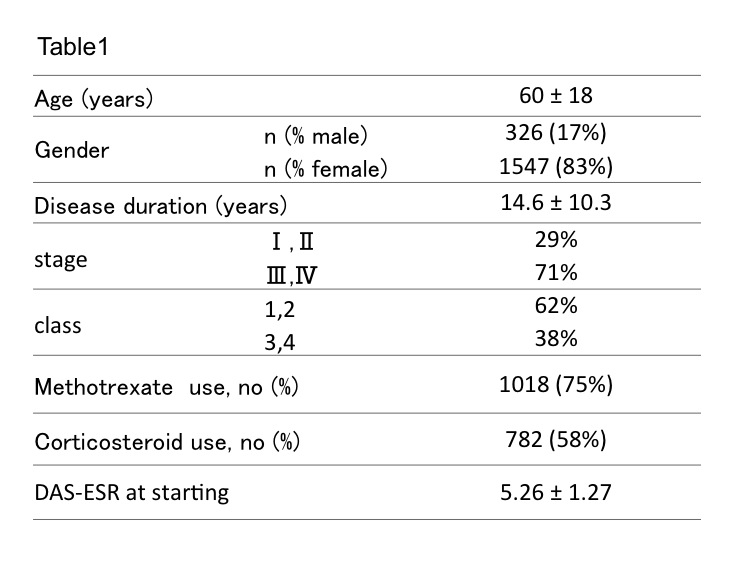
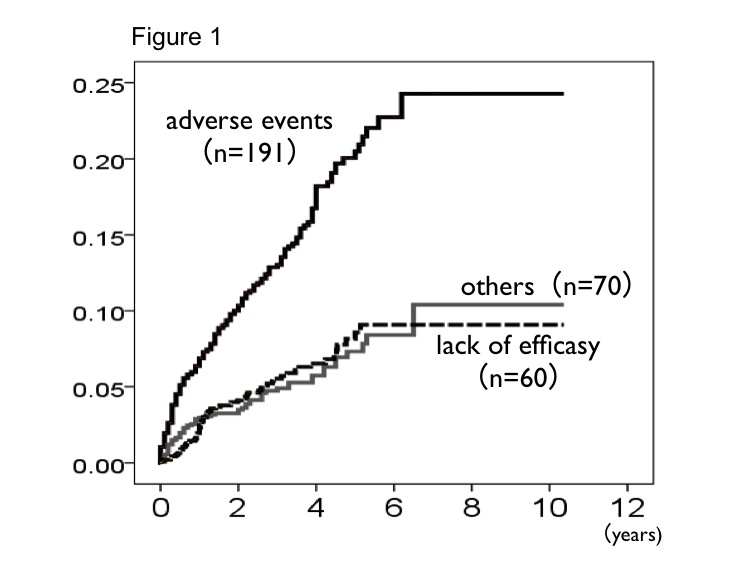
In total 1966 patients, 1479 patients were administered biologics continuously, 487 patients were withdrew. Table 1 showed the demographic date in total patients. The retention rate was 72% (n=1563) at least 1 year from starting biologics treatment, 68.7% (n=866) at 3 years, 65.6% (n=360) at 5 years. In 327 patients who were confirmed the reasons of discontinuation, the reasons were adverse events in 191 patients, lack of effectiveness in 66 patients, others in 70 patients. Comparison of incidence for discontinuation using cumulative hazard function, the reason of adverse events was significantly higher than others reasons (Figure 1). To identify o the risks of discontinuation, we used cox hazard model regression in patients who discontinued treatment due to adverse events and lack of effectiveness, the risk factors were over 70 years of age (OR 1.80 [1.31-2.46]), male (OR 1.79 [1.27-2.52]), over 3 of steinblocker class (OR 1.51 [1.12-2.04]). Non concomitant with methotrexate (OR 1.47 [1.06-2.04]).
Conclusion
The most common reason for discontinuation was adverse events. In a numerically greater proportion of patients who discontinued any biologic agent treatment, timing of discontinuation was within a first year from starting treatment. In addition, the risk factors for discontinuation were similar with those of adverse events.
Disclosure:
K. Terabe,
None;
T. Kojima,
Takeda Pharma Corporation, Janssen Pharmaceutical, and Astellas Pharma Corporation.,
2,
Mitsubishi Tanabe Pharma Corporation, Takeda Pharma Corporation, Eisai Pharma Corporation, Abbvie, Bristol-Myers Squibb、Pfizer and Chugai Pharma Corporation,
8;
N. Takahashi,
Abbott Japan Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Co. Ltd, Chugai Pharmaceutical Co. Ltd., and Bristol-Myers Squibb Co. Ltd. ,
8;
K. Funahashi,
Abbott Japan Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Co. Ltd, Chugai Pharmaceutical Co. Ltd., and Bristol-Myers Squibb Co. Ltd. ,
8;
A. Kaneko,
Janssen Pharmaceutica Product, L.P.,
8,
Astellas Pharma,
8,
Mitsubishi-Tanabe Pharma,
8,
Chugai,
8,
Eisai,
8,
Abbott Immunology Pharmaceuticals,
8,
Bristol-Myers Squibb,
8;
Y. Hirano,
AbbVie Inc.; Eisai Co. Ltd.; Mitsubishi Tanabe Pharma Corporation; Takeda Pharma Corporation; Pfizer Co. Ltd; Chugai Pharmaceutical Co. Ltd. and Bristol-Myers Squibb Co. Ltd.,
8;
Y. Kanayama,
Astellas Pharma,
8,
Eisai,
8,
Mitsubishi Tanabe Pharma Corporation,
8,
AbbVie Inc,
8,
Chugai,
8;
Y. Yabe,
Abbott Immunology Pharmaceuticals,
8,
Mitsbishi-Tanabe Pharma,
8,
Eisai,
8,
Chugai,
8,
Bristol-Myers Squibb,
8,
Pfizer Inc,
8;
N. Ishiguro,
AbbVie, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi Tanabe, Pfizer and Takeda.,
5,
AbbVie, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi Tanabe, Pfizer and Takeda.,
8.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reasons-and-risk-factor-for-discontinuation-of-biologic-agents-in-rheumatoid-arthritis-patients/