Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: JAK inhibitors (JAKi) have been a major development in treatment for many patients with RA due to their ability to block multiple cytokine pathways involved in pathogenesis. In Australia, government subsidisation enables rheumatologists to prescribe whichever b/tsDMARD they consider most clinically appropriate for individual patients. There has been rapid uptake of the 3 approved JAKi: tofacitinib (TOF; available since October 2015), baricitinib (BARI, Sept 2018) and upadacitinib (UPA; May 2020).
The objective of this study is to describe time to switch, switching patterns, concomitant csDMARDs, and reason for switch, in patients who initiated TOF, BARI or UPA as first-line, second-line, or third-line therapy and subsequently switched to an alternative JAKi, TNFi or other bDMARD (IL-6i, CTLA-4, and CD20).
Methods: The OPAL dataset is a collection of deidentified, aggregated clinical data derived from the electronic medical records of 112 rheumatologists in Australia. Data is captured at the point of care in custom-built software (Audit4, S4S Pty Ltd, Sydney, Australia) which also serves as the clinician’s prescribing software.1
Patients were eligible if they were aged 18-95 years with a physician diagnosis of RA and initiated a JAKi between 1 Oct 2015 and 30 Sept 2021. Data were summarised using descriptive statistics. Persistence to drug was analysed for the overall population of JAKi initiators using Kaplan-Meier analysis, with discontinuation of treatment analysed as the event.
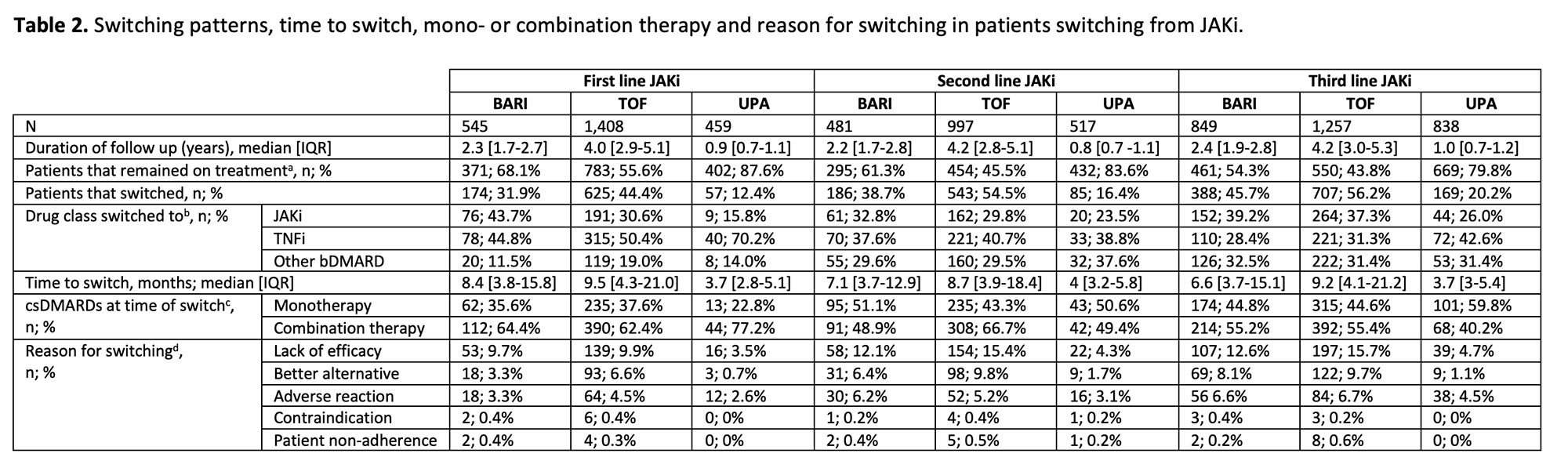
Results: 5,900 patients initiated a JAKi during the study window and were included in this analysis (BARI n=1,875, TOF n=3,662, UPA n=1,814). In the overall population, median persistence was comparable for BARI, TOF and UPA where there was sufficient follow up, and persistence was longest for patients that initiated a JAKi in first line therapy and shorter in patients receiving a JAKi as second line and third line therapy (Table 1). JAKi to JAKi switching was observed across all lines of therapy, however patients switching from first line TOF and UPA were more likely to switch to a TNFi than an alternative JAKi (Table 2). In later lines of therapy switching from JAKi to TNFi was less frequently observed, and bDMARDs with other mechanisms of action were more commonly utilised. At the time of switching from first line, 22.8% of patients were receiving UPA as monotherapy compared to 35.6% and 37.6% of BARI and TOF treated patients, respectively. The use of monotherapy increased in second and third line across all agents. ‘Lack of efficacy’ was the most common reason for switching, and a small proportion of patients initiating a JAKi experienced an adverse reaction prompting discontinuation of treatment. Cautious interpretation of UPA results is required due to limited duration of follow up.
Conclusion: In Australian real-world clinical practice, median persistence was similar across treatment groups and JAKi to JAKi switching was frequently observed.
b Denominator is total patients who switched from that JAKi.
c Defined as JAKi with at least one csDMARD (methotrexate, leflunomide, hydroxychloroquine, sulfasalazine, cyclosporine) at switch, regardless of corticosteroids.
d Denominator is total patients that initiated that JAKi.
To cite this abstract in AMA style:
Ciciriello S, Littlejohn G, Treuer T, Gibson K, Youssef P, Bird P, OSullivan C, Smith T, Deakin C. Real-world Utilisation and Switching Between Janus Kinase Inhibitors in Patients with Rheumatoid Arthritis in the Australian OPAL Dataset [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/real-world-utilisation-and-switching-between-janus-kinase-inhibitors-in-patients-with-rheumatoid-arthritis-in-the-australian-opal-dataset/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-utilisation-and-switching-between-janus-kinase-inhibitors-in-patients-with-rheumatoid-arthritis-in-the-australian-opal-dataset/