Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab is a fully human anti–interleukin-17A monoclonal antibody approved for the treatment of patients with moderate to severe ankylosing spondylitis (AS). A limited number of studies have evaluated the use of secukinumab in a real-world setting since its approval in the United States on January 15, 2016. The objective of this analysis was to describe the demographic and clinical characteristics of patients with AS who were treated with secukinumab in routine clinical practice, stratified by previous biologic experience.
Methods: Retrospective data from the Symphony Health Solutions Lx commercial claims database were used to identify patients with AS who had ≥ 1 secukinumab treatment between January 15, 2016 and December 31, 2016. Patients eligible for inclusion were ≥ 18 years of age who had the diagnosis of AS (ICD-10-CM Code M45 or M08.1; ICD-9-CM Code 720.0) and ≥ 1 pharmacy or medical claim in the 12 months prior to their first secukinumab treatment (index date), and any pharmacy or medical claim > 30 days after the index date. Patient demographics and secukinumab dosage were examined at the index date. Clinical characteristics, comorbidities and treatment history in the 12 months prior to the index date were identified and presented for biologic-naïve and biologic-experienced patients with AS.
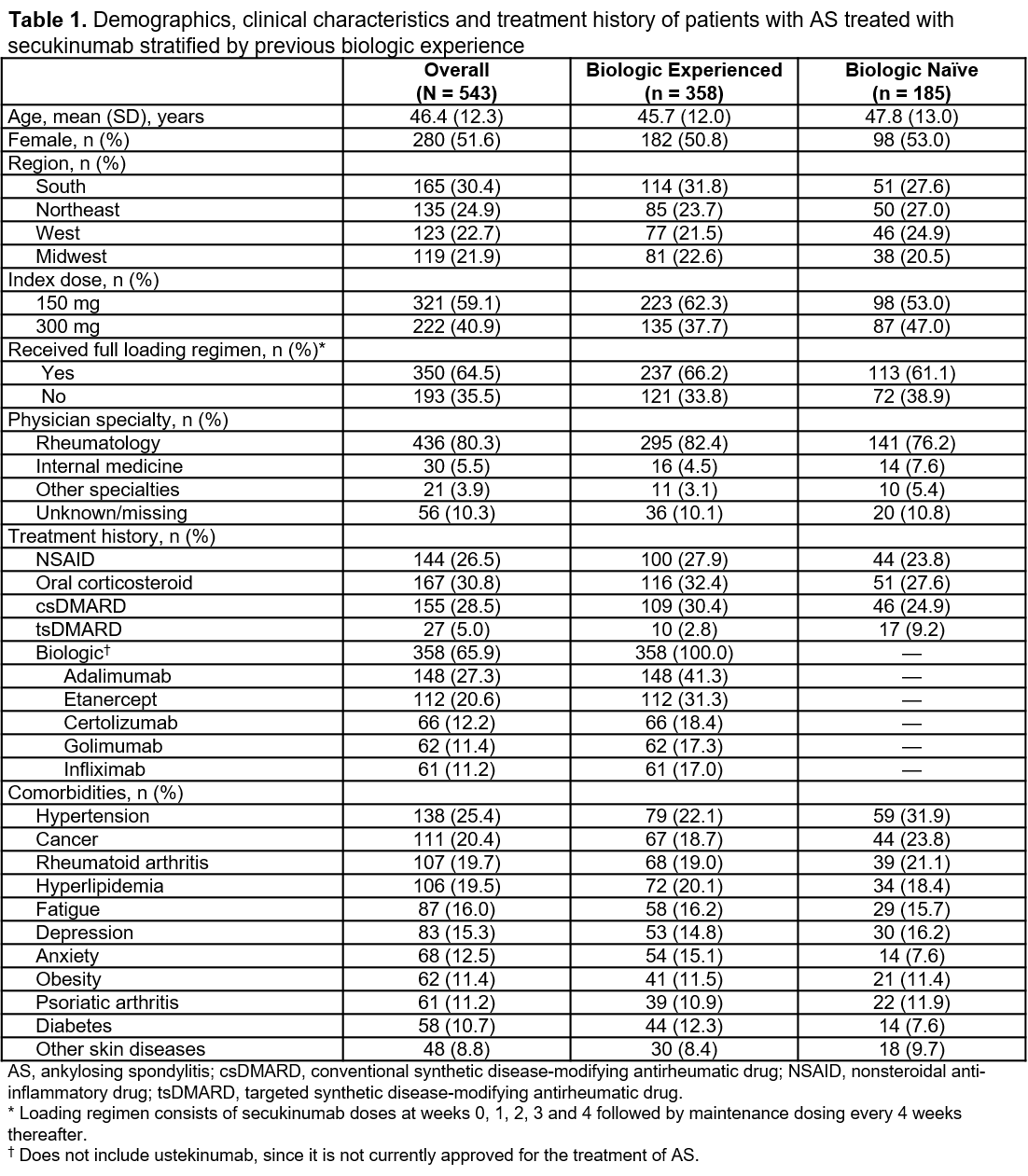
Results: A total of 543 patients who initiated secukinumab were included in this study; the mean (SD) age of included patients was 46.4 (12.3) years and 51.6% were female. Of the 543 patients, 358 patients (65.9%) had received another biologic in the previous 12 months, and 185 patients (34.1%) were biologic naïve. Patient demographics, clinical characteristics and treatment history were mostly similar between the two cohorts (Table 1). Most patients (59.1%) initiated secukinumab with the 150-mg dose (biologic experienced, 62.3%; biologic naïve, 53.0%); nearly two-thirds of all patients (64.5%) received a full loading regimen. A higher proportion of biologic-naïve patients had prior tsDMARD use compared with biologic-experienced patients (9.2% vs 2.8%); however, both cohorts were similar in prior NSAID, oral corticosteroid and csDMARD use. Among biologic-experienced patients, the most common biologics previously used were adalimumab (41.3%) and etanercept (31.3%). Overall, the most prevalent comorbidities were hypertension (25.4%), cancer (20.4%), rheumatoid arthritis (19.7%) and hyperlipidemia (19.5%).
Conclusion: In this retrospective, administrative claims-based study, approximately two-thirds of patients with AS who initiated secukinumab used biologics in the previous 12-month period. While most patients initiated secukinumab at the approved dose of 150 mg, nearly 40% of patients initiated secukinumab at a dose of 300 mg. These results provide early insights into real-world use of secukinumab among biologic-naïve and biologic-experienced patients with AS in the United States.
To cite this abstract in AMA style:
Oelke KR, Garg R, Li Y, Liu X, Zhou H, Park Y. Real-World Use of Secukinumab Among Biologic-NaïVe and Biologic-Experienced Patients with Ankylosing Spondylitis in the United States [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/real-world-use-of-secukinumab-among-biologic-naive-and-biologic-experienced-patients-with-ankylosing-spondylitis-in-the-united-states/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-use-of-secukinumab-among-biologic-naive-and-biologic-experienced-patients-with-ankylosing-spondylitis-in-the-united-states/