Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Apremilast (APR), an oral phosphodiesterase 4 inhibitor, demonstrated efficacy in adult patients with active PsA despite prior use of csDMARDs and/or biologics in phase III studies of PsA (Kavanaugh. Ann Rheum Dis. 2014; Cutolo. J Rheumatol. 2016; Edwards. Ann Rheum Dis. 2016). Although APR has been studied in combination with csDMARDs, APR in combination with biologic therapies has not been studied. The objective of this analysis was to understand the characteristics at treatment initiation of US patients with PsA in the real-world setting who are prescribed APR + biologic therapy and to compare them with patients treated with biologic monotherapy and patients treated with biologic therapy + csDMARDs.
Methods: Adult patients diagnosed with PsA and enrolled in the Corrona PsA/Spondyloarthritis Registry who received APR + biologic therapy, biologic monotherapy, or biologic therapy + csDMARDs for PsA from March 2013 to March 2019 were included. For biologic monotherapy, the index date was defined as the date of the treatment initiation visit. For combination therapy, the index date was defined as the date of the treatment initiation visit for the second/add-on drug. Patient demographics, clinical/treatment characteristics, disease activity, and physician- and patient-reported outcomes at the index date were summarized using descriptive statistics for continuous (mean [SD], median [interquartile range]) and categorical (n [%]) variables.
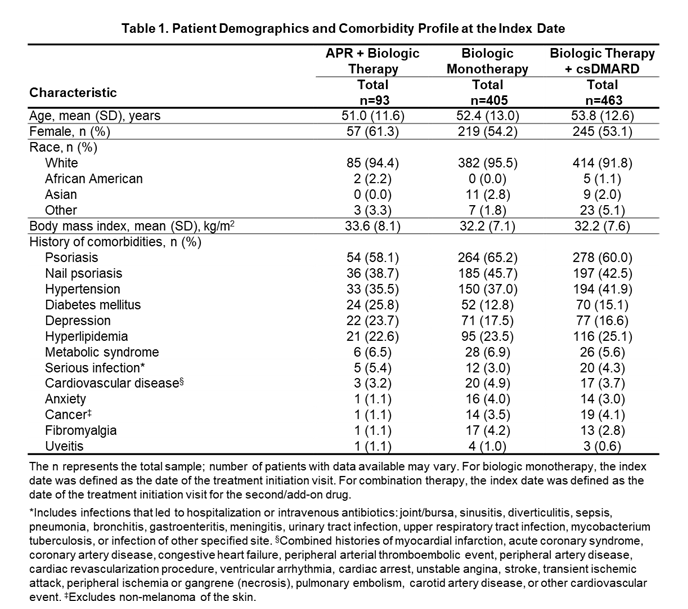
Results: In all, 961 patients were included in the analysis (APR + biologic: n=93; biologic monotherapy: n=405; biologic therapy + csDMARDs: n=463). Mean age was generally comparable across groups (Table 1). A greater proportion of females were treated with APR + biologic vs biologic monotherapy and biologic + csDMARDs (Table 1). Comorbidity history was generally similar among the groups, but the proportion of patients with depression and diabetes was numerically greater in the APR + biologic group and the proportion of patients with hypertension was numerically greater in the biologic + csDMARD group vs the other groups. The proportion of patients with comorbid psoriasis and nail psoriasis was highest in the biologic monotherapy group (Table 1). Mean duration of PsA was comparable across groups (Table 2). The majority (90.3%) of the APR + biologic group had received prior biologic therapy (Table 2). Disease activity was generally similar among groups based on mean cDAPSA score, proportion of patients in DAS28 remission, mean SJC (0-66) and TJC (0-68), presence of enthesitis and dactylitis, SPARCC enthesitis score, and mean dactylitis count, although fewer patients in the APR + biologic group had minimal disease activity (MDA) vs the other groups (Table 2). Mean PhGA and PtGA, as well as HAQ-DI, and pain VAS scores were similar among groups.
Conclusion: The majority of US patients who received APR + biologic were previously exposed to biologics. Patients initiating APR + biologic had similar characteristics vs those initiating biologic monotherapy and biologic + csDMARDs; however, fewer were in MDA at the time of initiation of the second/add-on drug. This suggests a greater use of APR + biologic combination in patients who previously were unable to achieve MDA.
To cite this abstract in AMA style:
Ogdie A, Liu M, Glynn M, Emeanuru K, Harrold L, Richter S, Guerette B, Mease P. Real-World Use of Apremilast in Combination with Biologic Therapy in Patients with Psoriatic Arthritis: Findings from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/real-world-use-of-apremilast-in-combination-with-biologic-therapy-in-patients-with-psoriatic-arthritis-findings-from-the-corrona-psoriatic-arthritis-spondyloarthritis-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-use-of-apremilast-in-combination-with-biologic-therapy-in-patients-with-psoriatic-arthritis-findings-from-the-corrona-psoriatic-arthritis-spondyloarthritis-registry/