Session Information
Date: Sunday, November 8, 2020
Title: Epidemiology & Public Health Poster III: Inflammatory Rheumatic Disease
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a systemic condition estimated to affect 0.05%-0.25% of the United States (US)1 population. With approval of ixekizumab (a selectively binding IL-17 inhibitor biologic) for treatment of PsA, there is need to understand its treatment patterns in the real world. The current study aimed to describe adherence, persistence, discontinuation, switching, and dosing among patients with PsA newly initiating ixekizumab (IXE).
Methods: The IBM MarketScan administrative claims databases were used to select adults (≥18 years) initiating IXE between 1/1/2016- 7/31/2019 for this retrospective study (earliest IXE claim = index). Eligible patients had >1 PsA diagnosis (ICD-9 696.0x or ICD-10 L40.50-L4.059) during the 12 months preceding index and remained continuously enrolled for 12 months following (follow-up period) index. Adherence (measured by proportion of days covered [PDC]) to IXE, persistence on IXE (< 60 day gap with sensitivity analysis using a < 45 and < 90 day gap), discontinuation of IXE (>90 day gap), and switching (discontinuation of IXE followed by a claim of a different biologic or targeted synthetic disease-modifying anti-rheumatic drug [tsDMARD] with ≥30 as monotherapy) patterns were reported over the 12-month follow-up period. IXE dose (calculated by multiplying the drug strength by the quantity and dividing by the days of supply for each claim) was reported during the starting/induction and maintenance phase for patients eligible for dosing calculations with and without comorbid psoriasis (PSO).
Results: There were 496 PsA patients initiating IXE eligible for analysis. Mean age was 51.1 (SD 9.8) years, 50.4% were female, and 93.4% had comorbid PsO. Most patients (91.9%) were biologic or tsDMARD experienced, and the average number of different biologic or tsDMARDs prior to starting IXE was 1.4 (SD 0.06). The most common drugs used prior to IXE were secukinumab, adalimumab, and ustekinumab (34.9%, 28.8%, and 21.4% respectively). Over the 12-month follow-up, 52.8% (using < 60-day gap) remained persistent (47.0% using < 45 day gap and 61.3% using < 90 day gap) on IXE (average of 262 days [SD 123] of persistence; Figure 1). Of all 496 IXE patients, 38.7% discontinued (>90 day gap), 13.5% had no other treatment, 4.6% restarted IXE, and 20.6% switched to a new biologic/tsDMARD within the 12-month follow-up period.
Adherence to IXE prior to discontinuation was high (mean PDC 0.85 [SD 0.12]) with 70.6% of patients identified as highly adherent (i.e. PDC >0.80) to therapy. Dose values during the starting/induction and maintenance periods were consistent with prescribing information for patients with and without comorbid PsO (Figure 2).
Conclusion: Results from this real-world analysis suggest that among PsA patients treated with ixekizumab, 91.9% had previously experienced inefficacy or intolerance to different biologics including other IL-17A and tsDMARD. 52.8% remained persistent and 20.6% switched to a new agent over 12 months of follow-up. Dosing is consistent with prescribing recommendations for patients with and without comorbid PsO.
 Figure 1: Time to Non-Persistence (60-day gap)
Figure 1: Time to Non-Persistence (60-day gap)
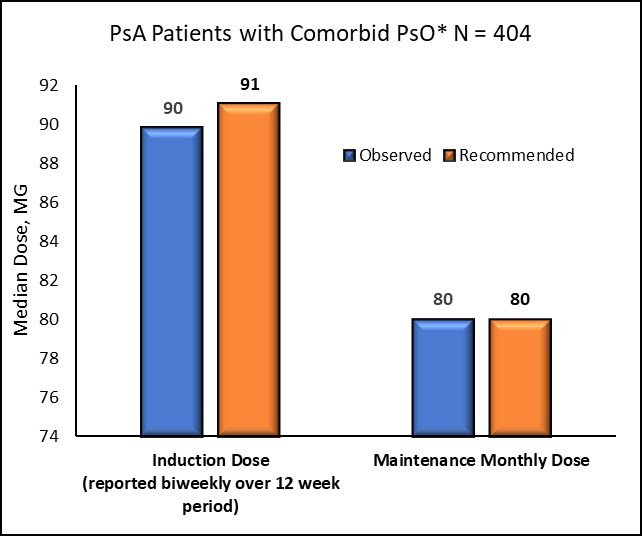 Figure 2a: IXE Dose* Observed in Real World Setting versus Recommended (based on prescribing information for FDA approved dose) Over 12 Months Following Initiation *For patients with comorbid PsO, dose was measured during an induction period of 12 weeks and then during maintenance starting at week 14 and continuing until discontinuation or end of the 12-month follow-up period. Recommended dose based on prescribing information for the FDA approved dose was defined as 160mg at week zero followed by 80mg at weeks 2,4,5,8,10 and 12 (which was equivalent to a dose of 91mg biweekly over the entire induction period).
Figure 2a: IXE Dose* Observed in Real World Setting versus Recommended (based on prescribing information for FDA approved dose) Over 12 Months Following Initiation *For patients with comorbid PsO, dose was measured during an induction period of 12 weeks and then during maintenance starting at week 14 and continuing until discontinuation or end of the 12-month follow-up period. Recommended dose based on prescribing information for the FDA approved dose was defined as 160mg at week zero followed by 80mg at weeks 2,4,5,8,10 and 12 (which was equivalent to a dose of 91mg biweekly over the entire induction period).
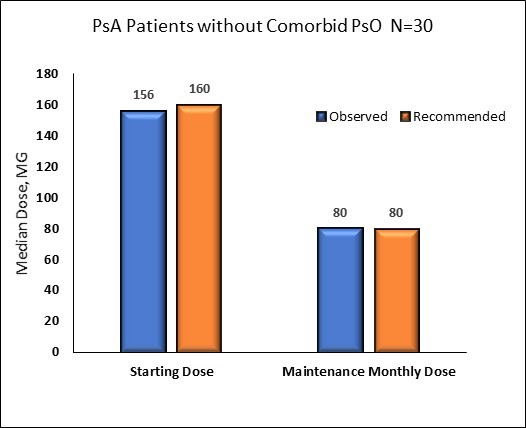 Figure 2b: IXE Dose* Observed in Real World Setting versus Recommended (based on prescribing information for FDA approved dose) Over 12 Months Following Initiation
Figure 2b: IXE Dose* Observed in Real World Setting versus Recommended (based on prescribing information for FDA approved dose) Over 12 Months Following Initiation
To cite this abstract in AMA style:
Murage M, Princic N, Park J, Malatestinic W, Zhu B, Atiya B, Kern S, Stenger K, Sprabery A, Ogdie A. Real World Treatment Patterns Among Patients with Psoriatic Arthritis Treated with Ixekizumab [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/real-world-treatment-patterns-among-patients-with-psoriatic-arthritis-treated-with-ixekizumab/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-treatment-patterns-among-patients-with-psoriatic-arthritis-treated-with-ixekizumab/
