Session Information
Date: Tuesday, October 23, 2018
Title: Pediatric Rheumatology – Clinical Poster III: Juvenile Idiopathic Arthritis and Uveitis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
The safety and efficacy of adalimumab (an anti-TNF-α antibody) treatment in patients with JIA have been demonstrated in clinical trials. This study was conducted to investigate the safety and effectiveness of adalimumab in patients with JIA in real-world settings in Japan.
Methods:
This all-case post-marketing surveillance (NCT01412021) was conducted at 108 centers in Japan. All patients receiving adalimumab treatment for JIA affecting multiple joints were enrolled between July 1, 2011 and February 26, 2016 and observed for 24 weeks and 1-/2-year follow-ups. The primary endpoint was incidence of adverse drug reactions (ADRs) and secondary endpoint was DAS28-4/ESR remission (<2.6) rate at each study time point.
Results:
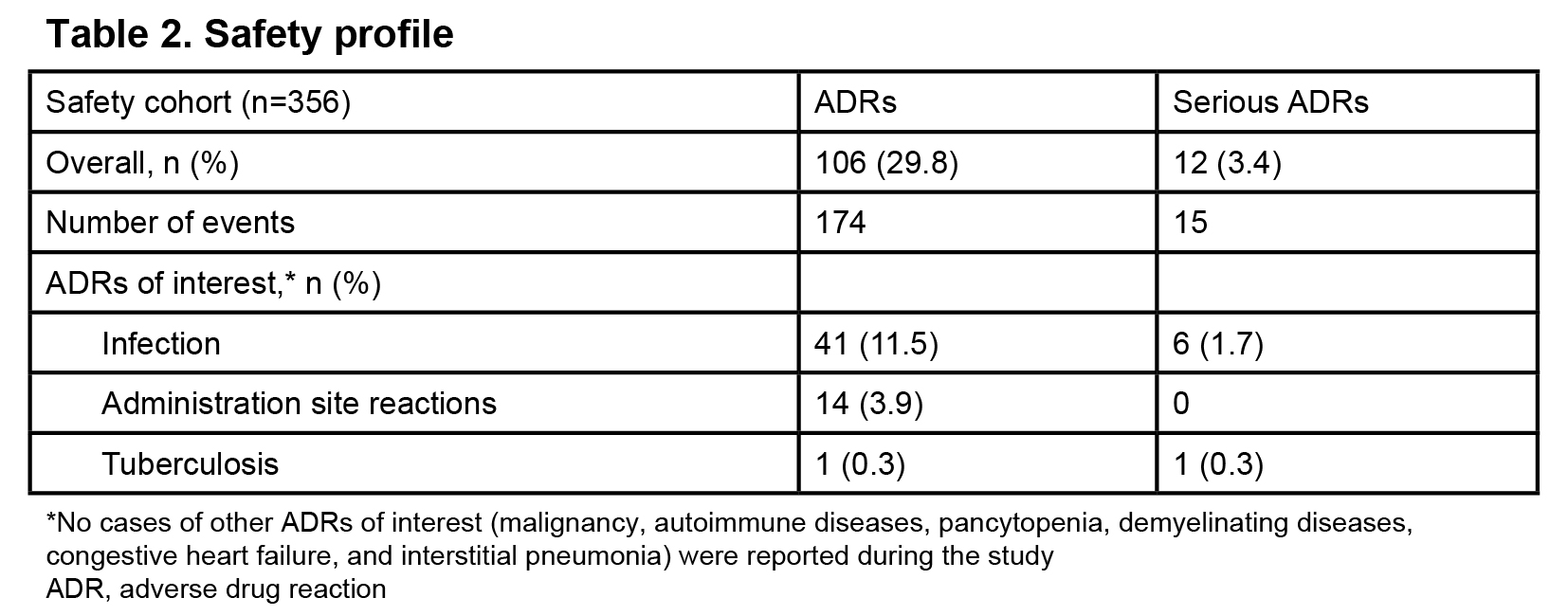
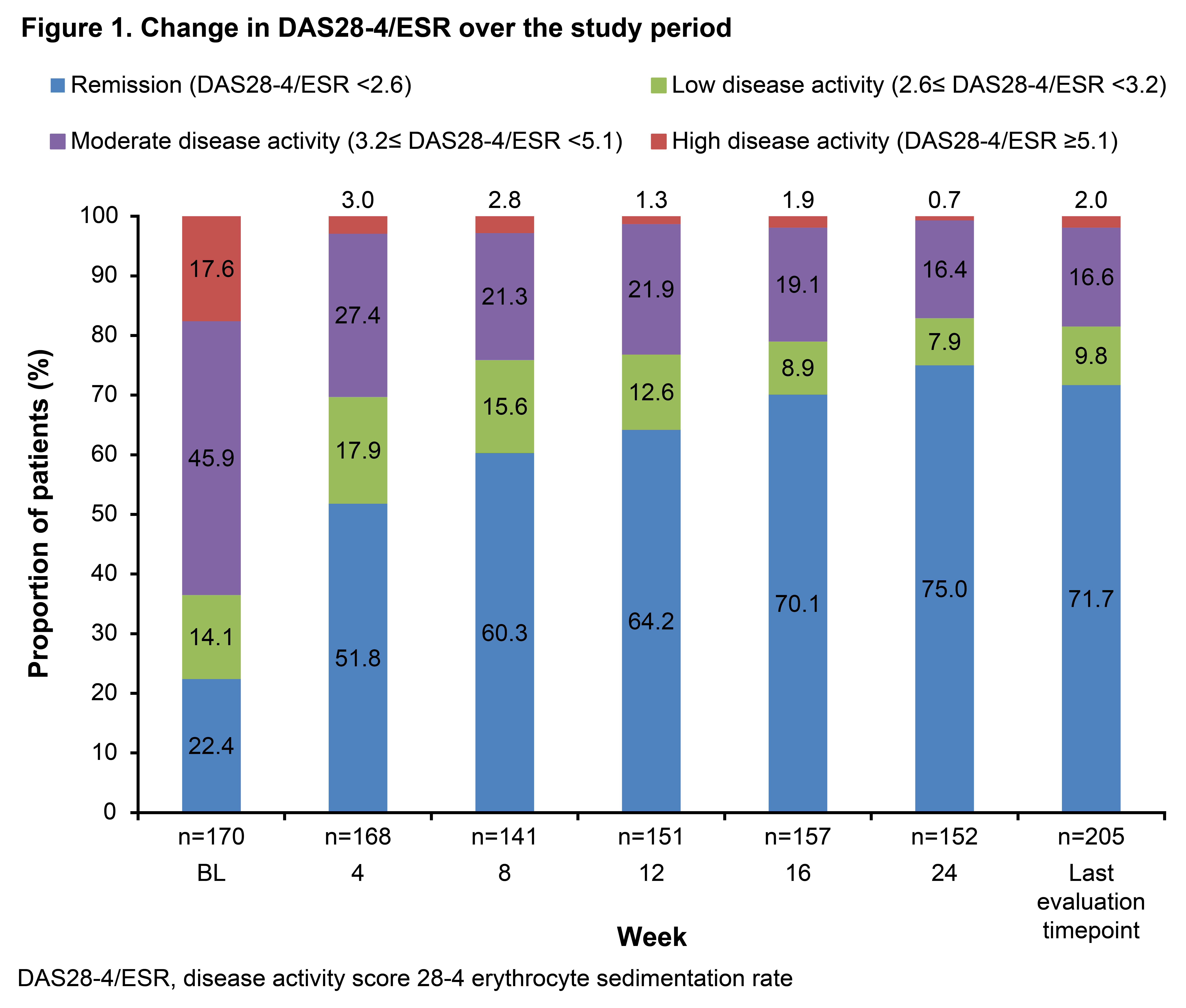
Of 375 patients enrolled, 356 were included in the safety cohort (Table 1). Adalimumab was administered at a dose of 20 mg every 2 weeks in 65 of 72 patients weighing 15 kg–<30 kg and at a dose of 40 mg in 236 of 240 patients weighing ≥30 kg. Mean (standard deviation) treatment duration was 159.0 (31.2) days and number of administration was 11.3 (3.8) times. Treatment was discontinued in 44 (12.4%) patients with the most common reason being ineffectiveness in 28 (7.9%), and the second reason as adverse event in 9 (2.5%) patients. Overall, 174 ADRs were reported in 106 (29.8%) patients (Table 2). No cases of malignancy were reported during the study and at the 1- and 2-year follow-ups. Incidence of ADRs gradually decreased from treatment initiation over time (duration [weeks], %: 0–4, 12.6%; 5–8, 7.1%; 9–12, 4.3%; 13–16, 2.4%; 17–20, 1.9%). Incidence of ADRs was numerically higher in children aged< 15 years (34.6% [79/228]) than in patients aged ≥15 years (21.1% [27/128]) and in those weighing 15–<30 kg (47.2% [34/72]) than in those <15 kg (12.5% [1/8]) and ≥30 kg (27.5% [66/240]). Self-administration errors were reported in 2 (0.8%) patients. In the effectiveness population (n=205), the DAS28-4/ESR remission rate increased from 22.4% (38/170 patients) at baseline to 75.0% (114/152 patients) at 24 weeks (Figure 1).
Conclusion:
In patients with JIA, adalimumab treatment was well tolerated with acceptable safety and effectiveness in real-world settings.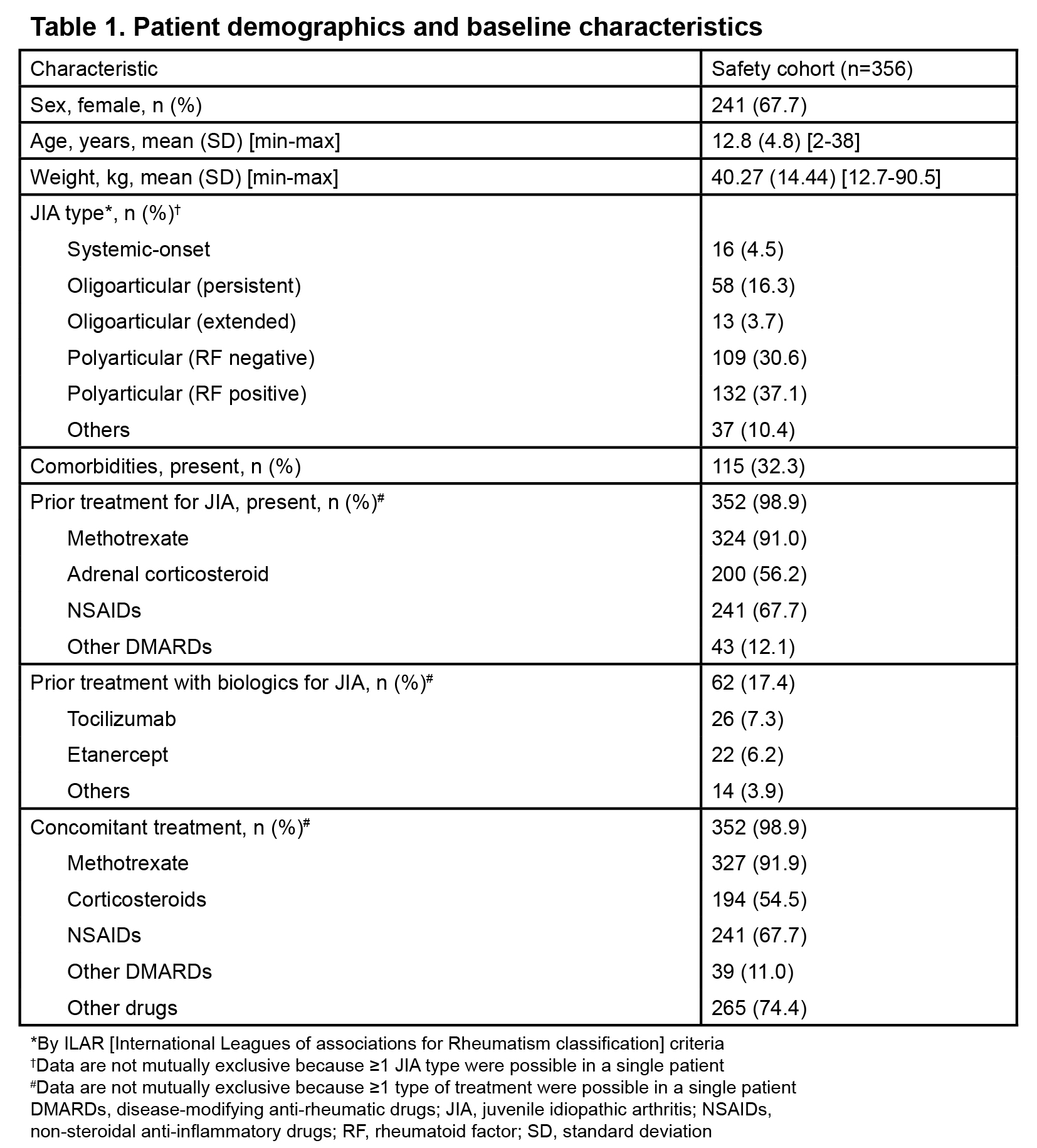
To cite this abstract in AMA style:
Takei S, Iwata N, Kobayashi I, Igarashi T, Yoshinaga Y, Matsubara N, Sunaga N, Ito A, Yokota S. Real-World Safety and Effectiveness of Adalimumab in Patients with Juvenile Idiopathic Arthritis: Results from a Post-Marketing Surveillance in Japan [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/real-world-safety-and-effectiveness-of-adalimumab-in-patients-with-juvenile-idiopathic-arthritis-results-from-a-post-marketing-surveillance-in-japan/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-safety-and-effectiveness-of-adalimumab-in-patients-with-juvenile-idiopathic-arthritis-results-from-a-post-marketing-surveillance-in-japan/
