Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
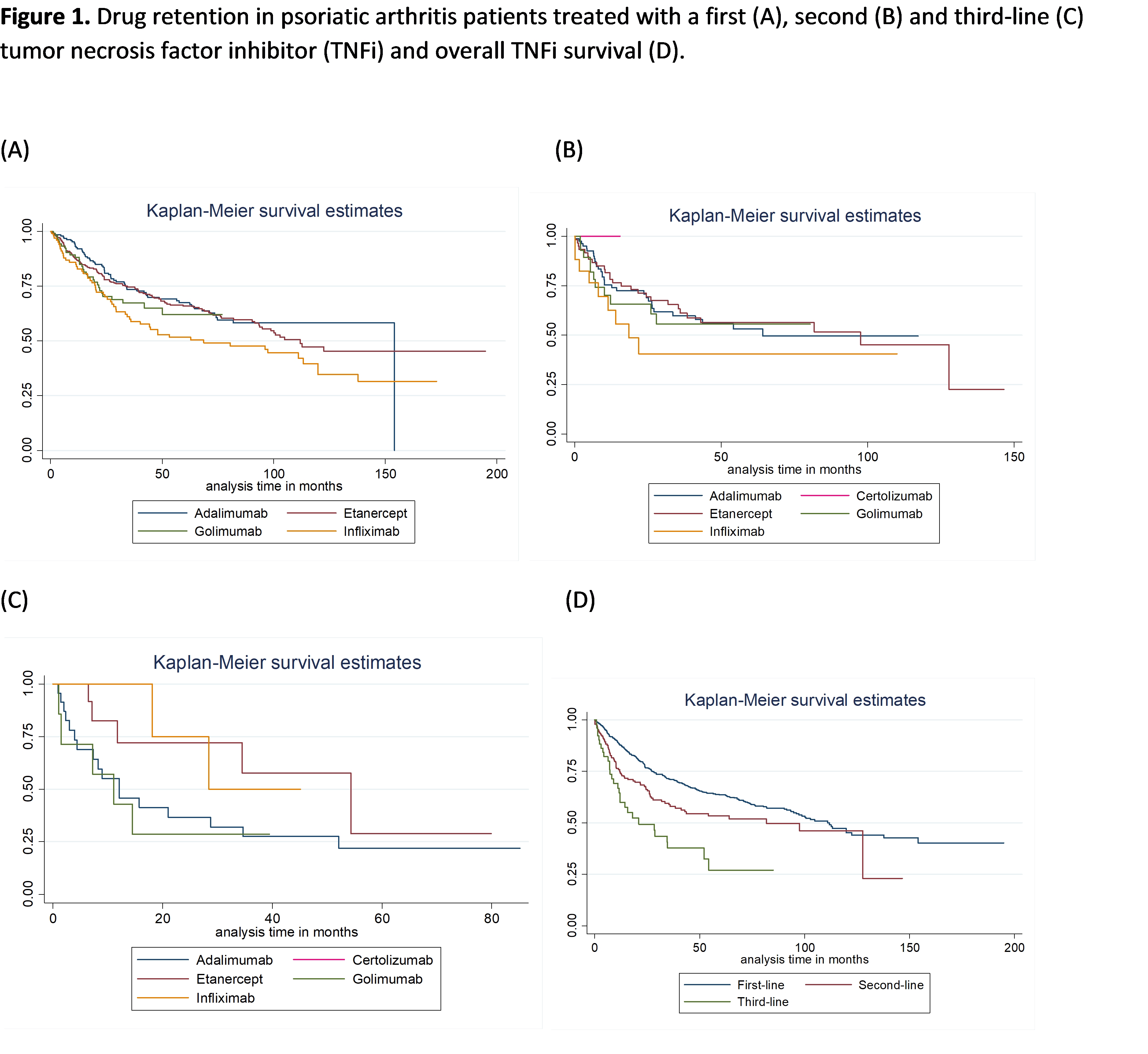
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) lead to a dramatic improvement in the management of psoriatic arthritis (PsA). Nevertheless, a significant proportion of patients do not respond or are intolerant to TNFis, requiring treatment switch for an adequate control of disease activity. The aim of this study is to assess long-term TNFis drug retention and predictors for TNFi discontinuation in PsA patients registered at Rheumatic Diseases Portuguese Registry (Reuma.pt).
Methods: All PsA patients registered at Reuma.pt, with at least one TNFi prescription were included. Drug retention for a 1st, 2nd and 3rd-line TNFi was assessed by Kaplan-Meier survival analysis. Comparative analysis of survival curves was performed using the Wilcox (Breslow) for the equality of survival functions. The reasons for TNFis discontinuation were described as frequencies. Predictors of discontinuation, were studied using a Cox model considering discontinuation of the first TNFi, independently of the indication.
Results: 750 PsA patients were identified, with a mean age of 47.6 years; 50.3% female. 185 patients (26.2%) were treated with adalimumab, 322 (45.7%) etanercept, 100 (14.2%) golimumab and 98 (13.9%) with infliximab as first TNFi. The overall retention of TNFi was 49±40 months when treated with a 1st TNFi, decreasing to 36±33 months for the 2nd TNFi, and 23±23 months for the 3rd TNFi. After being treated with a 1st TNFi, 35.8% discontinued therapy, 53.5% due to lack or loss of effectiveness and 24.4 % due to adverse events. The rates of discontinuation for the 2nd and 3rd TNFi were of 39% and 54%, respectively, with similar proportions for lack/loss of effectiveness and adverse events for the 2nd (62.3%; 21.6%) and 3rd TNFi (63.0%; 22.2%). When considering predictors of discontinuation for the first TNFi, independently of the indication, being female increased by 2.1 times the risk of discontinuation (HR=2.1, p-value=0.003). In addition, each increase of one unit in DA28 at baseline, increased the risk of discontinuation by 18% (HR=1.18, p-value=0.039). Finally, being treated with infliximab in comparison with etanercept, increased by 2.0 times the risk of discontinuation, assuming the other variables as constant (HR=2.0, p-value=0.007, global p-value=0.012).
Conclusion: The overall persistence of a 1st TNFi was high in PsA patients registered at Reuma.pt, decreasing on average 13 months, in those who switched to a 2nd TNFi or a 3rd TNFi. Lack or loss of response were the main reasons for TNFi discontinuation, independently of TNFi position. Female gender and higher peripheral disease activity (DAS28) at baseline, were associated with an increased risk of discontinuation of TNFis. Likewise, Infliximab doubled the risk of discontinuation when compared with etanercept.
Financial support for statistics and report writing was provided by Novartis, S.A.
To cite this abstract in AMA style:
Vieira-Sousa E, Eusébio M, Ávila-Ribeiro P, Khmelinskii N, Machado AR, Martins-Rocha T, Bernardes M, Santos Faria D, Leite Silva J, Santos H, Miguel C, Carvalho P, Costa T, Teixeira L, Meirinhos T, Nero P, Santos MJ. Real-World Long-Term Effectiveness of Switching between Tumor Necrosis Factor Inhibitors in Psoriatic Arthritis Patients from the Rheumatic Diseases Portuguese Register [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/real-world-long-term-effectiveness-of-switching-between-tumor-necrosis-factor-inhibitors-in-psoriatic-arthritis-patients-from-the-rheumatic-diseases-portuguese-register/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-long-term-effectiveness-of-switching-between-tumor-necrosis-factor-inhibitors-in-psoriatic-arthritis-patients-from-the-rheumatic-diseases-portuguese-register/